Sansure’s New Immunoassay Product Kicks off a New Era of Eelectrochemiluminescence Immunoassay

Recently, Sansure Biotech officially launched a new immunoassay product – Accucise Enhanced Electrochemiluminescence Platform at its 15th Anniversary Celebration. Under the joint witness of more than 2,000 attendees, a new era of electrochemiluminescence (ECL) immunoassay was opened.

Dr. Yin Peng, the Senior Vice President and Chief Technology Officer of Sansure Biotech and the Chairman and General Manager of Accucise Diagnostics, unveiled the new immunoassay product.
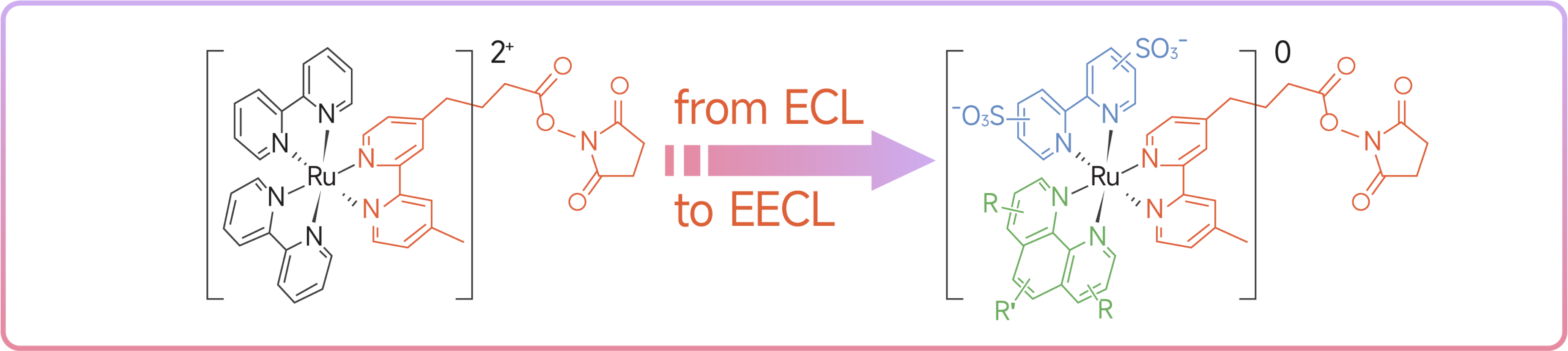
In contrast to the traditional ECL technology using tris (2,2′‐bipyridine) ruthenium derivatives as markers, Sansure’s Enhanced Electrochemiluminescence (EECL) technology has been improved in three aspects: luminescent markers, magnetic bead coupling method, and electrochemical regulation. The improved EECL technology features more intense luminescent signals, lower noise, more flexible sensitivity, shorter response time, and greater anti-interference capability, yielding a more precise response system.
1. Tris-heteroleptic electronically neutral ruthenium complexes (NRC) are used as luminescent markers
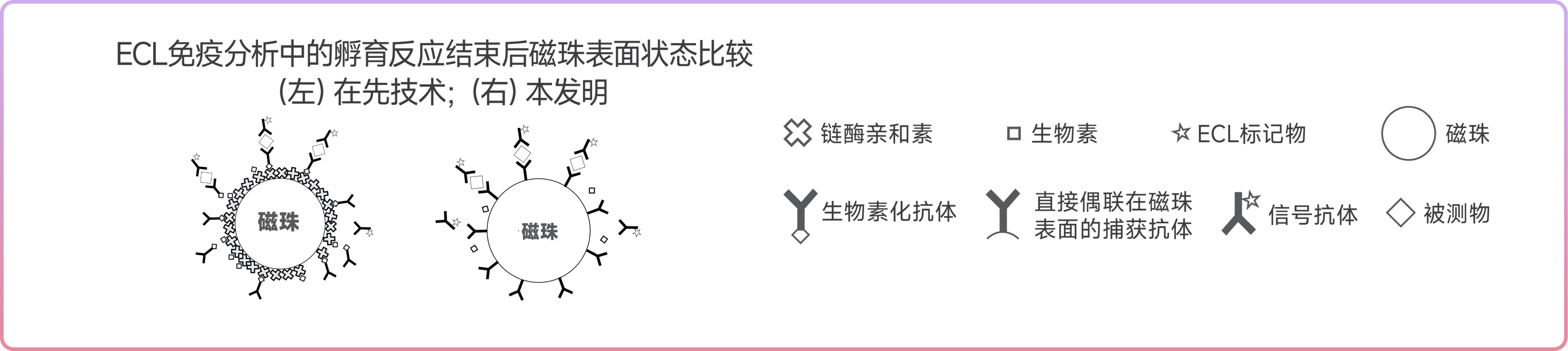
2. The detection process is completely free from interference of biotin and streptavidin antibodies in samples
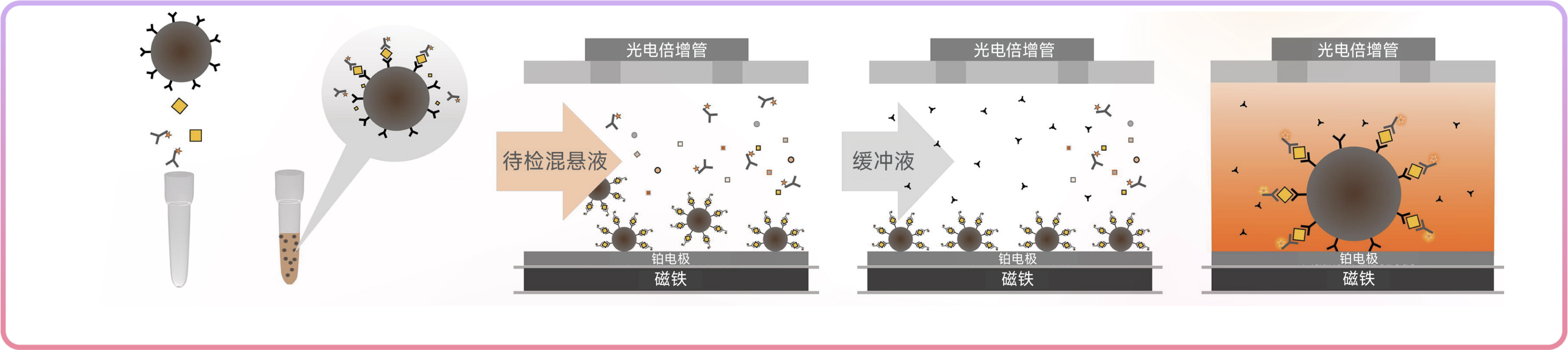
3. Rapid detection, with a response time of 5 min and the first result within 8 min
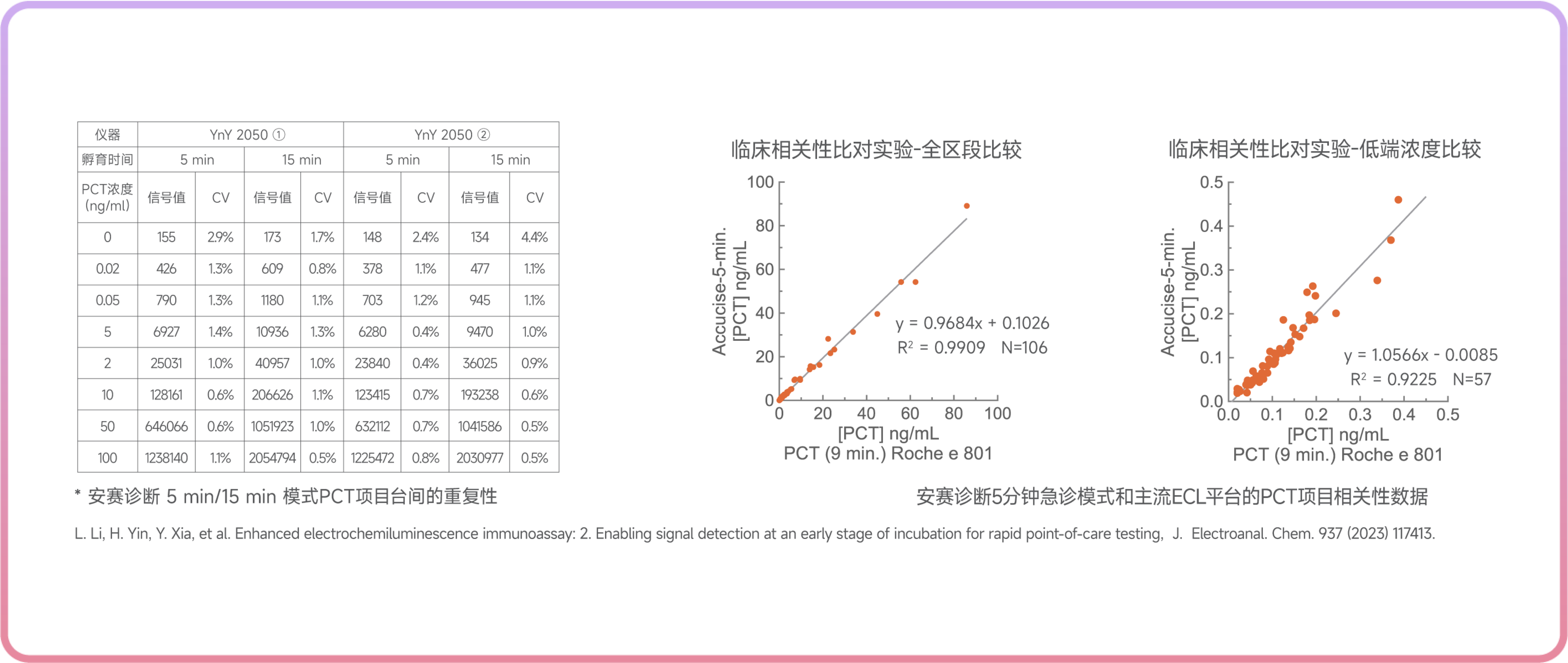
Innovation delivers a more precise response system:
In 2023, Sansure Biotech entered the electrochemiluminescence (ECL) immunoassay track with Accucise Diagnostics and launched a series of new products in succession. In the future, Sansure Biotech intends to optimize product performance and customer experience by focusing on its products and brand to open up a new era of ECL immunoassay.





