-

iPonatic III Pro – Portable Molecular Diagnostic System
mPOCTBrief
To provide time-saving, labor-saving and safe solutions for efficient nucleic acid analysis is always the intention of research and development for Sansure iPonatic series product. The iPonatic III Pro combines the core technical advantages of the previous generations of iPonatic series products, integrates better fluid controlling, magnetic beads method for nucleic acid extraction and PCR process, to avoid the traditional PCR experimental process time-consuming, complex operation and other drawbacks. By truly realizing the "sample in, result out" fully automated gene detection, and with Sansure’s flexible and extended testing menu, iPonatic III Pro is the ultimate choice for efficient molecular diagnostics.Features
- Simplicity: Fully automated DNA/RNA extraction and detection to minimize manual labor
- Precision: Features fast PCR with real-time data analysis for reliable results
- Scalability: Manages up to 8 devices with iScreen, meeting diverse testing demands
- Speed: Delivers results within approximately 1 hour, ensuring rapid TAT time
- Compatibility: Processes various pathogens across different Viral Transport Media
- Intelligence: Offers wireless LIS and IoT connectivity for advanced disease surveillance
Parameters
Model S-Q37A (with ultrasonic function) S-Q37B (without ultrasonic function) Dimension About 400mm * 141mm * 401mm (L*W*H) WeightAbout 9.9kgFunctions Nucleic acid extraction, amplification & detection, data analysis Sample process Liquid transfer, heating and ultrasonic funciton (S-Q37A only) for DNA purification Analysis Support melting curve analysis Channels FAM, VIC, ROX, CY5 Maximum heating rate 10°C/sec Maximum cooling rate 3°C/sec Temperature accuracy 0.5°C Display Built-in 7-inch high-definition touch screen Communication USB2.0, RJ45, Type-C, Wi-Fi, Bluetooth, LIS_LH7 Input voltage 100~240VAC Power frequency 50/60Hz Rated power 140VA Operating temperature 10°C~30°C Operating humidity 20%RH~85%RH, non-condensing Barometric pressure 85.0kPa~106.0kPa Altitude Less than 3,000 m Transportation and storage Environment temperature range: -20°C~55°C Relative humidity range: ≤85%RH, non-condensing Test menu
Respiratory:- Six RP (Influenza A virus, Influenza B virus, Respiratory syncytial virus, Adenovirus, Human rhinovirus and Mycoplasma pneumonia)
- 6LRP (Klebsiella pneumoniae, Streptococcus pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa, Legionella pneumophila and Staphylococcus aureus)
- SARS-CoV-2/Flu/RSV (SARS-CoV-2, Influenza virus A/B (Flu) and Respiratory syncytial virus (RSV))
- COVID (ORF1ab and N genes of novel coronavirus (2019-nCoV))
- SARS-CoV-2/ FluA/B (SARS-CoV-2, Influenza A and Influenza B)
- MP (Mycoplasma pneumoniae)
- MERS (HCoV-MERS)
- TB and RIF (Mycobacterium tuberculosis and Rifampicin resistance mutations)
- HPV 13+2 (Human papillomavirus (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68))
- MG/MH/TV (Mycoplasma genitalium, Mycoplasma hominis, and Trichomonas vaginalis)
- EV/EV71/CA16 (Enterovirus, Coxsackievirus A16 and Enterovirus 71)
- GBS (Group B streptococcus)
- DENV/ZIKV/CHIKV (Dengue virus (DENV), Zika virus (ZIKV), and Chikungunya virus (CHIKV) RNA)
Use scenarios
Hospital, Nursing Home, Airport, Cruise, CDC, Clinic, Lab, Pharmacy, etc.
*All use scenarios must comply with local regulations.Product video
https://youtu.be/oGEpTLWrCIU?si=WUtry-NhRyFGeSup -


iPonatic III – Portable Molecular Workstation
mPOCTBrief
iPonatic III - Portable Molecular Workstation is a new member of Sansure's iPonatic series, optimizing molecular diagnostics with its advanced capabilities. With its cutting-edge technology, iPonatic III ushers in a new "digital and intelligent" era of molecular diagnostics, empowering healthcare professionals and providing rapid and accurate results.Features
- Fully automated rapid testing process and “sample-in-result-out” system make test results available in 15 - 45 minutes.
- Pre-packaged kits significantly shorten the hands-on time and reduce the chance of contamination.
- Rich and extendable testing menu meets the testing demands of different pathogens from every scenario.
- Through innovative wireless connection modules, large smart screen, and flexible combination abilities, samples can be tested anytime upon arrival.
- Only 7.2 kilograms (15.84 Pounds)
- Benefiting from state-of-the-art “SanUI” interactive system and HD smart touch screen, the test results will be displayed directly.
- Top international industrial design standard embodies Sansure’s distinctive design concepts.
Parameters
Model S-Q36ADimension 391 mm×140 mm×368 mm (L×W×H) WeightAbout 7.2kgChannelsFAM, VIC/HEX, ROX/Texas Red, CY5Duration15 - 45min (SARS-CoV-2)LOD 200 copies/mL (SARS-CoV-2)Maximum heating rate≥10℃/secMaximum cooling rate≥3℃/secTemperature accuracy±0.5°CFunctionsNucleic acid extraction, amplification detection, data analysisDisplayBuilt-in 7-inch high-definition touch screen, 12.1-inch smart screen (optional)Interfaces/communicationUSB2.0, RJ45, Type-C, WI-FI, Bluetooth, LIS_LH7 Input voltag100-240 VACPower frequency50/60HzRated power160VATemperatureOperating conditions:10°C- 30°CTransportation and storage: -40°C- 55°CHumidityOperating conditions: 30% - 80%, non-condensingTransportation and storage: ≤ 93%, non-condensingBarometric pressure85.0kPa - 106.0k PaAltitudeLess than 3,000 mQualificationCETest menu
RTI: SARS-CoV-2 (ORF1ab, N gene) SARS-CoV-2 (ORF1ab, N gene, E gene) SARS-CoV-2/Flu A/Flu B SARS-CoV-2/Flu/RSV Six Respiratory Pathogens (Flu A/Flu B/RSV/AdV/HRV/MP) Acinetobacter baumannii and Canidia albican (AB/CA) Flu A/Flu B Mycoplasma Pneumoniae (MP) Mycobacterium Tuberculosis (TB) Bordetella Pertussis (BP) Respiratory Syncytial Virus (RSV) Streptococcus Pneumoniae (SP) Carbapenemase Gene (KPC) MERS Legionella pneumophila (Lp) Adenovirus (AdV) STI & HPV: HPV 13+2 (Identifies HPV 16 and HPV 18, reports 13 other high risk types in pooled results) HPV 15 HR (Reports 15 high risk HPV types in pooled results) HPV 16&18 HPV 6&11 HSV-2 HSV-1&2 Mycoplasma Genitalium/Mycoplasma Hominis/Trichomonas Vaginalis (MG/MH/TV) Neisseria Gonorrhoeae (NG) Ureaplasma Urealyticum (UU) Mycoplasma Genitalium (MG) Chlamydia Trachomatis/Ureaplasma Urealyticum/Neisseria Gonorrhoeae(CT/UU/NG) Other infections: Epstein-Barr Virus (EBV) Group B Streptococcus (GBS) Toxigenic Clostridium difficile (CD) * Monkeypox Virus (MPXV) *Use scenarios
Medical laboratories, emergency rooms, fever clinics, remote areas, CDCs, airports, customs, etc.
*All use scenarios must comply with local regulations. -


iPonatic – Portable Molecule Workstation
mPOCT, PCR InstrumentsBrief
Innovations in molecular assays—especially on the point-of-care testing (POCT) front—have spread this testing from molecular diagnostics laboratories into clinical microbiology laboratories—and now into general laboratories and even clinics and exam rooms.[1] Access to sensitive and rapid infectious disease diagnostic assays is essential for accurate diagnosis, effective treatment, and timely infection control, making POCT vital to reducing TAT. With novel POCT on the horizon, future studies are warranted to determine cost savings, antimicrobial usage, TAT, patient impact, and how to best implement in non-microbiology clinical laboratories and clinics.[2] Sansure iPonatic (Portable Molecule Workstation) aims to innovate traditional diagnostic mode and assist precision diagnosis. It can provide quickly and convenient diagnosis experience for clinical emergency, health management, military safety, biological emergency and other applications.Core technologies
- Rapid nucleic acid lysis at room temperature within 1 minute
- Ultra fast amplification system in 8-45 minutes
- Integrated automatic data analysis software
- Results immediately printed by built-in printer
Performance
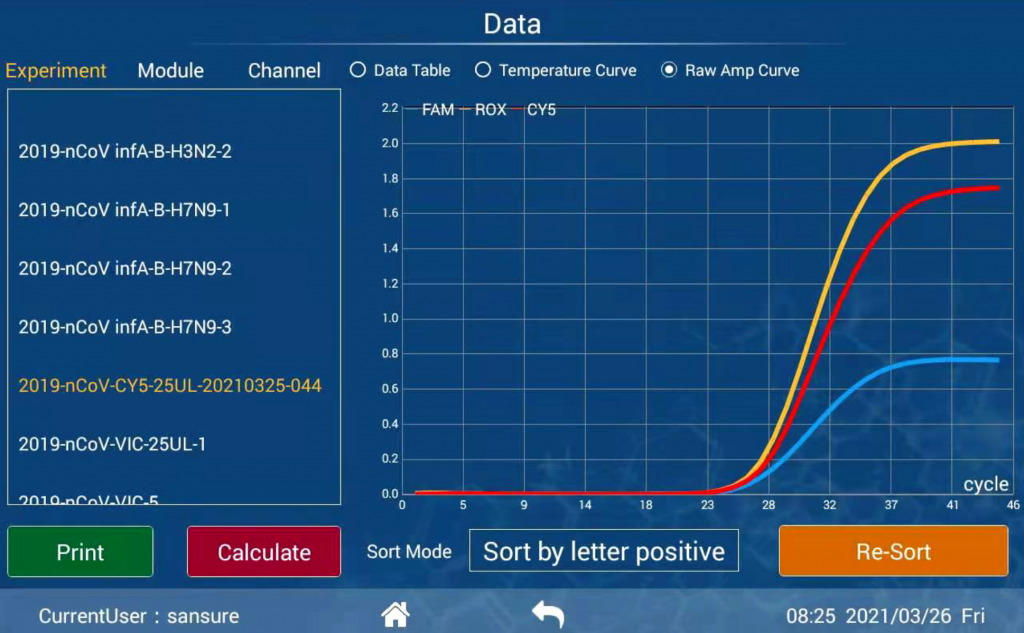
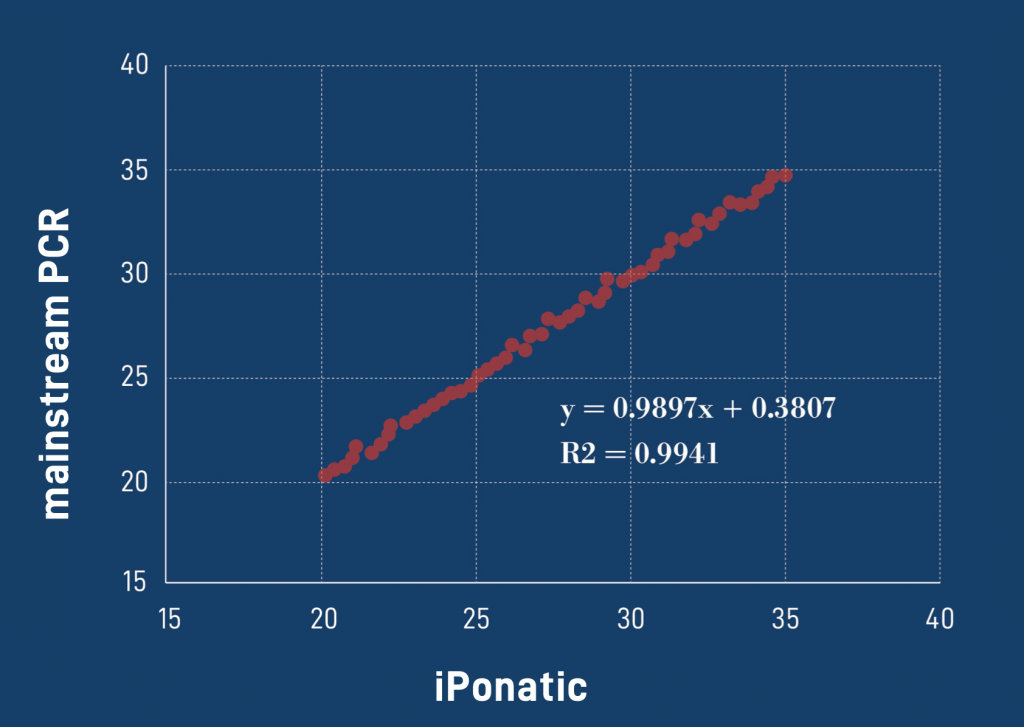
Diagnostic results consistent with international mainstream PCR instruments
Parameters
Model S-Q31A S-Q31B Detection platform Real-time PCR Detection module 1 amplification module 4 amplification modules Temperature control Liquid metal coated ceramic heating / Air cooling technology Heating rate ≥6.0℃ /s (from 50℃ to 95℃ ) Cooling rate ≥2.0℃ /s (from 95℃ to 50℃ ) Excitation light source LED Detector High sensitivity photodiode Applicable dyes FAM VIC ROX CY5 Sensitivity Can detect single copy gene Electrical specification AC 100-200V 50/60Hz Dimension 230×284×376 mm (L×W×H) 336.5 × 280 × 435 mm (L×W×H) Weight 9.7Kg 13Kg Qualification CE Test menu
Respiratory Infections : SARS-CoV-2, SARS-CoV-2/FluA/B, MP, ADV, BP.... HPV Infections : HrHPV, HPV 13+2, HPV 16/18, HPV 6/11.... Children's health : EBV, HCMV.... STIs : CT/UU/NG, CT, UU, NG, HSV-2.... Other test projects are under developmentReferences
[1]. Kozel TR, Burnham-Marusich AR. Point-of-care testing for infectious diseases: Past, present, and future. J Clin Microbiol 2017;55:2313-20.
[2]. Paige M.K. Larkin, Omai B. Garner. Molecular Point-of-Care Testing in Clinical Laboratories Laboratorians can lead a new era in rapid testing with expertise in quality control and result interpretation. Clinical Laboratory News. JUL.1.2020
