-


S3014E HCMV – Human Cytomegalovirus DNA Quantitative Fluorescence Diagnostic Kit
Other InfectionsBrief
Human cytomegalovirus (HCMV), also called cell inclusion body virus, is a double helix DNA virus and belongs to β genus of herpes virus family, which causes infected cells to enlarge and has a huge intranuclear inclusion. The ways of infection of HCMV is mainly through contact, blood transfusion, intrauterine and birth canal, and the infections are commonly found in fetus, newborns, pregnant women, etc. If the pregnant women are infected, it may cause their newborns congenital monstrosity. When the organism immune deficiency or immune system is under inhibitory state, people can be easily infected by HCMV, such as the patients receiving immunosuppressive therapy after transplantation of organ, the patients receiving malignant tumor chemotherapy and AIDS patients, etc. If these patients are infected by HCMV, it usually causes high mortality and serious diseases. Clinical tests suggest that HCMV infection hasn’t specific manifestations and it causes harm to multiple organs, especially the liver and lung. A statistical analysis of positive and negative rate of HCMV in various kinds of clinical indications shows HCMV infection is probably related with various diseases, such as Cytomegalovirus hepatitis, Infant hepatitis syndrome, liver dysfunction, pneumonia, Bronchitis, Upper respiratory tract infections, enteritis, enterocolitis, diarrhea, hematemesis, heart failure, etc. The Human Cytomegalovirus DNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of HCMV DNA in human urine, serum and peripheral blood samples. It is intended for use as an aid in the diagnosis of an HCMV infection and for observing drug efficacy.Parameters
Product features Parameter Specimen Type urine, serum, and peripheral blood. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen HCMV Internal Control cloning plasmid PCR Instrument ABI7500, Roche LC 480, Stratagene Mx3000P, SLAN-96P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T Qualification CE -


S3015E EBV – Epstein-Barr virus DNA Quantitative Fluorescence Diagnostic Kit
Other InfectionsBrief
Epstein-barr virus (EBV) is known to be the first virus that is definitely related with human tumors. EBV infection mainly causes infectious mononucleosis in children and tumor-related diseases such as Burkitt’s lymphoma, lymphoid tissue hyperplasia in immunocompromised individuals, primary lymphoma, empyema associated lymphoma, T-cell lymphoma, NK cell lymphoma/leukemia, Hodgkin’s disease, nasopharyngeal carcinoma, stomach cancer, lymphoid epithelial tissue cancer, smooth muscle tumors, etc. The Epstein-Barr virus DNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the quantification of Epstein-Barr Virus (EBV) DNA in peripheral blood. Test result can be used as an aid in the diagnosis of an EBV infection and in observation of drug efficacy.Parameters
Product features Parameter Specimen Type peripheral blood. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen EBV Internal Control cloning plasmid PCR Instrument ABI7500, Roche LC 480 and Stratagene Mx3000P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T Qualification CE -

C003E BCI – Nucleic Acid Test Kit for HBV, HCV, HIV (Type1+2)
Blood-borne Infections (BBIs)Brief
Nucleic Acid Test Kit for HBV, HCV, HIV (Type1+2), is based on real-time fluorescence PCR technology and used for nucleic acid qualitative detection of HBV, HCV, HIV1+2 in plasma. This kit is intended for use as a blood donor screening test to detect HBV DNA, HCV RNA, HIV-1 RNA and HIV-2 RNA in pooled or individual sample from healthy blood donors, blood donors of various components (red blood cells, platelets and plasma) and other types of blood donors. All plasma to be tested can be screened as individual samples or tested in pools after mixing with each equal aliquots according to routine serological screening results HBV, HCV and HIV samples. For the pooled sample showed positive test results, carry out individual testing, the individual test result should be used as the final test result of this sample. The test results of this diagnostic kit can distinguish reactivity of between HBV, HCV and HIV.Advantages
High sensitivity- HBV 3IU/mL
- HCV 10IU/mL
- HIV 45IU/mL
- 576 tests/ 5h(pooling)
- 45 tests / 4.5h(single
- HBV A-H
- HCV 1-6
- HIV-1(M/N/O)and HIV-2
- Four tests for a tube of samples
- Direct discriminating positive
- Extraction and amplification process
- No need to be on duty
- One extractor + one amplifier
- Less consumable material consumption
Features
Sample preparation and extraction in one module Fully automatic sample preparation and nucleic acid extraction in one module to build up a integrated reaction system Flexible testing mode 6 pooling sample testing or individual donor testing (IDT) supported Advanced magnetic beads technology Nanometer-level beads enable beads-in-PCR amplification ensuring maximum nucleic acid template Patented magnetic beads lateral suction technology Thorough waste liquid removal allows minimal magnetic beads loss Sophisticated sample information processing Automatic recognition of the sample barcode & sample tracking and archiving table generation available High throughput 558/45 samples result output in one time Minimal system maintenance time Less than 20 minutes startup with fewer maintenance tasks Excellent accuracy A total number of 105,124 blood bags which confirmed as negative by serological testing were tested against some referential screening kits. 15 false-negative results in reference tests were found.Parameters
Qualification CE -

S3034E HCV Genotype – Hepatitis C Virus Genotype Diagnostic Kit
Blood-borne Infections (BBIs)Brief
By applying real-time PCR technology, the Hepatitis C Virus Genotype Diagnostic Kit (PCR-Fluorescence Probing) is designed for qualitative identification of HCV genotypes (including genotypes 1b, 1, 2, 3, 4, 5 and 6) from HCV RNA positive samples. The test results can be used as an aid in the identification of HCV genotypes and determination of an appropriate therapeutic treatment indicated for the above listed gentoypes. The results can be used only for clinical reference, and cannot be used as the only evidence for adjusting therapeutic drugs. Clinical symptoms and other laboratory test results should also be considered to comprehensively determine the patients treatment.Parameters
Product features Parameter Specimen Type serum Extraction Platform Magnetic beads technologies Genotype 1b, 1(1b, 1a), 2 (2a), 3 (3a, 3b), 4 (4a), 5 (5a), 6 (6a) Internal Control Plasmid PCR Instrument Mx3000P,Slan 96P Sensitivity 1000 IU/mL Spec. 12T Qualification CE -


S3119E HCV Ultra – Hepatitis C Virus RNA Quantitative Fluorescence Diagnostic Kit
Blood-borne Infections (BBIs)Brief
Hepatitis C Virus RNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the quantification of HCV RNA in human serum or plasma. It is intended for use as an aid in the diagnosis of an HCV infection and observing drug efficacy. Hepatitis C is mainly caused by HCV infection and transmitted through blood. Chronic infection of HCV can lead to chronic inflammation of liver and fibrosis, and some patients may develop into liver cirrhosis, even Hepatocellular Carcinoma (HCC). It has huge harms on patients’ health and life quality, and has become a severe social and public health issue.Parameters
Product features Parameter Specimen Type Serum or plasma Extraction Platform Advanced magnetic beads technology Genotype HCV 1-6 genotype Internal Control Lentivirus particles PCR Instrument SLAN-96P, ABI7500, Roche cobas 480 Sensitivity 12 IU/mL Linear range 25—1.0E+08 IU/mL Spec. 24T -

S3013E HBV fast – Hepatitis B Virus DNA Quantitative Fluorescence Diagnostic Kit
Blood-borne Infections (BBIs)Brief
Hepatitis B Viral (HBV) DNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the quantification of HBV DNA in human serum or plasma. It can be used to evaluate antiviral treatment and monitor the therapeutic effect by monitoring HBV DNA baseline levels and changes in patient blood. Test results should not be taken as the only indicator for evaluation of diseases, but to be combined with patients clinical symptoms and other laboratory tests to analyze the diseases.Parameters
Product features Parameter Specimen Type Serum or plasma Extraction Platform One-tube fast test release Genotype HBV genotype A-H Internal Control Plasmid Compatible Instrument ABI7300 , Stratagene Mx3000P , Roche Light Cycler 480 , ABI7500, SLAN-96P and QuantStudio 5 Sensitivity 30 IU/mL Linear range 100—5.0E+09 IU/mL Spec. 24T, 48T -

S3118E HBV Ultra – Hepatitis B Virus DNA Quantitative Fluorescence Diagnostic Kit
Blood-borne Infections (BBIs)Brief
Hepatitis B Virus DNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) (HBV Ultra) is an in vitro nucleic acid amplification test for the quantification of Human HBV DNA in human serum or plasma. It is intended for use as an aid in diagnosing an HBV infection and observing drug efficacy.Performance
Advanced magnetic beads technology HBV DNA detection and viral load measurement are essential for treatment decisions and patient monitoring. Sansure's HBV Ultra uses the advanced magnetic beads technology to extract HBV DNA from clinical Plasma. Our technology achieved high sensitivity and wide linear range detection to meet of clinical diagnosis and follow-up needs. Highly conservative primer probe design Sansure primers and probes target select the highly conservative S gene of HBV, which can cover A-H genotypes and avoid missed detection. High-efficient quality control system HBV Ultra uses UNG enzyme + dUTP system to remove carry-over contamination to avoid a false positive result. HBV Ultra uses Internal Control HBV Ultra uses Internal Control is the full name to whole-process the HBV extraction and amplification process to avoid false negative results.Parameters
Product features Parameter Specimen Type Serum or plasma Extraction Platform Advanced magnetic beads technology Genotype HBV genotype A-H Internal Control Pseudoviruses PCR Instrument SLAN-96P, ABI7500, Roche cobas 480 Sensitivity 5 IU/mL Linear range 20—2.0E+09 IU/mL Spec. 48T -

S3017E HPV 6,11 – Human papillomavirus (Type 6 and 11) DNA Fluorescence Diagnostic Kit
HPV InfectionsBrief
HPV (Type 6 and 11) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of low-risk HPV (type 6/11) DNA in reproductive tract secretions. It is intended for use as an aid in the diagnosis of an HPV (type 6/11) infection and patients with clinically suspected genital warts.Parameters
Product features Parameter Specimen Type reproductive tract secretions Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 6, 11 Internal Control β--globin gene PCR Instrument ABI7500,QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II,iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -

S3019E HPV 16, 18 – Human papillomavirus (Type 16 and 18) DNA Fluorescence Diagnostic Kit
HPV InfectionsBrief
HPV (Type 16 and 18) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (Type 16 and 18) DNA in reproductive tract secretions. It is intended for use as an aid in the diagnosis of an HPV (Type 16 and 18) infection, and the early screening of cervical cancer.Parameters
Product features Parameter Specimen Type reproductive tract secretions Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 16, 18 Internal Control β--globin gene PCR Instrument ABI7500,QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II,iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -

S3360E HPV 15HR – 15 High-risk Human Papillomavirus Nucleic Acid Diagnostic Kit
HPV InfectionsBrief
15 High-risk HPV DNA Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68) present in exfoliated cells from females’ cervix. The test results can be used as an aid in the diagnosis of a high-risk HPV infection.Parameters
Product features Parameter Specimen Type exfoliated cells from females’ cervix Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68 Internal Control β--globin gene PCR Instrument ABI7500, QuantStudio 5, MA-6000, SLAN-96P, LightCycler 480, Stratagene Mx3000P, iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 1000 copies/mL Spec. 48T, 12-P Qualification CE -


S3027E HPV G15 – High-risk Human Papillomavirus DNA (Genotype) Diagnostic Kit
HPV InfectionsBrief
This High-risk HPV DNA (Genotype) Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68) present in exfoliated cells from females’ cervix. The test results can be used for identification of HPV genotypes.Parameters
Product features Parameter Specimen Type Exfoliated cells from females’ cervix Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68 Internal Control β--globin gene PCR Instrument SLAN-96P, ABI 7500, Roche LC 480 and QuantStudioTM 5 , Stratagene Mx3000P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 24T/48T Qualification CE -


S3057E HPV 13+2 – Human Papillomavirus DNA Diagnostic Kit
HPV InfectionsBrief
Base on the study results by WHO International Agency for Research on Cancer (IARC) and other international organizations, the 13 kinds of genotypes including HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 are classified as high-risk HPV, and the 5 kinds of genotypes including HPV26, 53, 66, 73, 82 are classified as medium-risk HPV. Human Papillomavirus DNA Diagnostic Kit (PCR-Fluorescence Probing) chooses the above 13 kinds of high-risk genotypes and two popular kinds of medium-risk genotypes that are HPV 53 and 66 to be the target genotypes, in order to guarantee that the kit is capable of cervical carcinoma screening and risk evaluation. Moreover, it is clearly indicated that the females at the age of 30 or above who have no abnormal cervical cytology but the HPV detection is positive, especially for HPV16 and HPV18 infected females, should get vaginoscopy immediately. Therefore, the diagnostic kit can be used for detection of the 15 kinds of target genotypes and also subtype identification of HPV16 and HPV18.Parameters
Product features Parameter Specimen Type Exfoliated cells from females’ cervix Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types Type 16+18+31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68 Internal Control β--globin gene PCR Instrument ABI7500, MA-6000, SLAN-96P, QuantGene 9600 , iPonatic S-Q31A&B, S-Q36A Amplification Time 70 min Sensitivity 200 copies/mL Spec. 48T, 24-P Qualification CE -

S3002E HSV-2 – Herpes Simplex Virus Type 2 DNA Fluorescence Diagnostic Kit
Reproductive Tract InfectionsBrief
Herpes Simplex Virus Type 2 (HSV-2) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is used to detect the presence of HSV-2- DNA in samples such as male urethral swab and female cervical swab. The detection result can be used as an aid in the diagnosis of an HSV-2 infection. The test provides a molecular diagnostics-based solution for early diagnosis of venereal disease and for preliminary screening of high-risk venereal disease groups.Parameters
Product features Parameter Specimen Type Genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen HSV-2 Internal Control Recombinant plasmid PCR Instrument ABI7500,QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II,iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -


S3004E UU – Ureaplasma Urealyticum DNA Fluorescence Diagnostic Kit
Reproductive Tract InfectionsBrief
The Ureaplasma Urealyticum (UU) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is used to detect the presence of UU-DNA in samples such as male urethra swab and female cervical swab. The detection result can be used as an aid in the diagnosis of a UU infection. The test provides a molecular diagnostics-based solution for early diagnosis of venereal disease and for preliminary screening of high-risk venereal disease groups.Parameters
Product features Parameter Specimen Type Genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen UU Internal Control Recombinant plasmid PCR Instrument ABI7500,QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II,iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -


S3003E NG – Neisseria Gonorrhoeae DNA Fluorescence Diagnostic Kit
Reproductive Tract InfectionsBrief
Neisseria Gonorrhoeae (NG) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is used to detect the presence of NG-DNA in samples such as male urethral swab and female cervical swab. The detection result can be used as an aid in the diagnosis of an NG infection. The test provides a molecular diagnostics-based solution for early diagnosis of venereal disease and for preliminary screening of high-risk venereal disease groups.Parameters
Product features Parameter Specimen Type Genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen NG Internal Control Recombinant plasmid PCR Instrument ABI7500, QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II, iPonatic S-Q31A&B, S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -

S3001E CT – Chlamydia Trachomatis DNA Fluorescence Diagnostic Kit
Reproductive Tract InfectionsBrief
Chlamydia Trachomatis (CT) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) applies to detect CT DNA in specimens like reproductive tract secretion. The detection result can be used for aiding diagnosis of CT infection, which provides molecular diagnosis base for early diagnosis of venereal disease and for preliminary screening of venereal disease high-risk groups.Parameters
Product features Parameter Specimen Type genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen CT Internal Control Recombinant plasmid PCR Instrument ABI 7500, Roche LC480,Stratagene Mx3000P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T Qualification CE -


S3050E CT/UU/NG – Chlamydia Trachomatis/Ureaplasma Urealyticum/Neisseria Gonorrhoeae DNA Diagnostic Kit
Reproductive Tract InfectionsBrief
Chlamydia Trachomatis/Ureaplasma Urealyticum/Neisseria Gonorrhoeae DNA Diagnostic Kit (PCR-Fluorescence Probing) is a qualitative in vitro test for simultaneous detection of Chlamydia Trachomatis, Ureaplasma Urealyticum and Neisseria Gonorrhoeae DNA in sterile calcium alginate swab specimens from male urinary tract secretion and female genital tract secretion by applying real-time fluorescence quantitative PCR technique, which can provide molecular diagnosis evidence for the early diagnosis of related sexually transmitted diseases and initial screening of high-risk STD population.Parameters
Product features Parameters Specimen Type genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen CT, UU, NG-DNA Internal Control β--globin gene PCR Instruments ABI7500,SLAN-96P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T Qualification CE -


S3113E SC2/FluA/B – SARS-CoV-2 and Influenza A/B Virus Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Most people infected with the COVID-19 virus will experience mild to moderate respiratory illness and recover without requiring special treatment. Elder people, and those with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop serious illness. Sansure COVID-19 diagnostic solutions are used to directly detect the presence of viral RNA, which will be detectable in patients before antibodies form or symptoms of the disease are present, which means the test results can tell whether or not that someone gets virus very early on in their illness. This kit can also joint-detect RNA of influenza A virus and influenza B virus.Parameters
Product features Parameters Specimen Type Oropharyngeal swab, sputum Extraction Platform One-tube fast release technology Advanced magnetic beads technology Target Genes SARS-CoV-2:ORF 1ab, N gene; influenza A:M gene; influenza B:NP gene Internal Control Rnase P gene PCR Instrument ABI7500, SLAN-96P, MA-6000, QuantGene 9600, iPonatic S-Q31A&B,S-Q36A Sensitivity 200 copies/mL Spec. 24T, 48T, 24-P Qualification CE -

S3148E SC2/Flu/RSV – SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus Multiple Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
The SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus Multiple Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) is a real-time RT-PCR test intended for the qualitative Diagnostic of nucleic acid from SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus Multiple in the nasopharyngeal swabs and oropharyngeal swabs from individuals.
As the seventh coronavirus that infects humans, the SARS-CoV-2 can cause fever, fatigue, dry cough, dyspnea and other symptoms. In severe cases, it can cause acute respiratory distress syndrome, septic shock, and even death. At the same time, the SARS-CoV-2 has a strong spreading ability and has a wide range. Influenza virus (Influenza virus) can cause acute respiratory infections, with clinical manifestations of fever, headache, myalgia, fatigue, rhinitis, sore throat and cough. Influenza viruses can aggravate underlying diseases (such as heart and lung diseases) or cause secondary bacterial pneumonia or primary influenza viral pneumonia. The elderly and people with various chronic diseases or physical weakness are prone to severe complications and mortality higher after infecting influenza. Respiratory syncytial virus (RSV) belongs to the Pneumovirus genus of the Paramyxoviridae family. It mainly causes lower respiratory tract infections such as bronchiolitis and pneumonia in infants under 6 months, as well as rhinitis , Cold and other upper respiratory tract infections in older children and adults.
Parameters
Product features Parameter Covering pathogensSARS-CoV-2, Influenza Virus and Respiratory Syncytial VirusSpecimen TypesNasopharyngeal swab and oropharyngeal swabTechnical PlatformOne-tube fast release technologyAdvanced magnetic beads technologyInternal ControlRNase P geneCompatible InstrumentABI 7500; MA-6000; SLAN®-96P; iPonatic S-Q31A/S-Q31B/S-Q36ASensitivity500 copies/mL.QualificationCE -


S3102E SC2 – Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
COVID-19 is an infectious disease caused by a newly discovered coronavirus named SARS-CoV2. Most people infected with the COVID-19 virus will experience mild to moderate respiratory illness and recover without requiring special treatment. Elder people, and those with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop serious illness. Sansure COVID-19 diagnostic solutions are used to directly detect the presence of viral RNA, which will be detectable in patients before antibodies form or symptoms of the disease are present, which means the test results can tell whether or not that someone gets virus very early on in their illness.Performance
- One-tube/fast release technology
- Up to 96 samples at one time
- Simple operation process, less specialist training
- Room temperature lysis, less contamination
- Sampling types : nasopharyngeal swab, oropharyngeal swab, alveolar lavage fluid, sputum, serum, whole blood ,Feces
- Enhance large-scale screening efficiency
- Internal control: human housekeeping gene RNase P
Parameters
Product features Parameters Specimen Type Nasopharyngeal swab, oropharyngeal swab, alveolar lavage fluid, sputum, serum, whole blood, feces Extraction Platform One-tube fast release technology Advanced magnetic beads technology Internal Control Rnase P gene PCR Instrument ABI7500, QuantStudioTM 5, SLAN-96P, MA-6000, Bio-Rad CFX-96, QuantGene 9600, LightCycler 480, iPonatic S-Q31A&B,S-Q36A Sensitivity 200 copies/mL Spec. 24T, 48T, 24-P Qualification CE -

S3121E SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay)
Respiratory Tract InfectionsBrief
SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay) is intended for the qualitative detection of the SARS-CoV-2/InFluA/InFluB nucleocapsid protein in human nasopharyngeal or oropharyngeal swabs. Test results will be available for reading in 15-20 minutes. Positive results indicate the presence of viral antigens. This kit is for in vitro diagnostic use.
Features
- Accurate LOD 80TCID50 /ml, Specificity 100%
- Convenient No instrument needed, easy to operate
- Fast Result available in only 15-20 min
Instruction
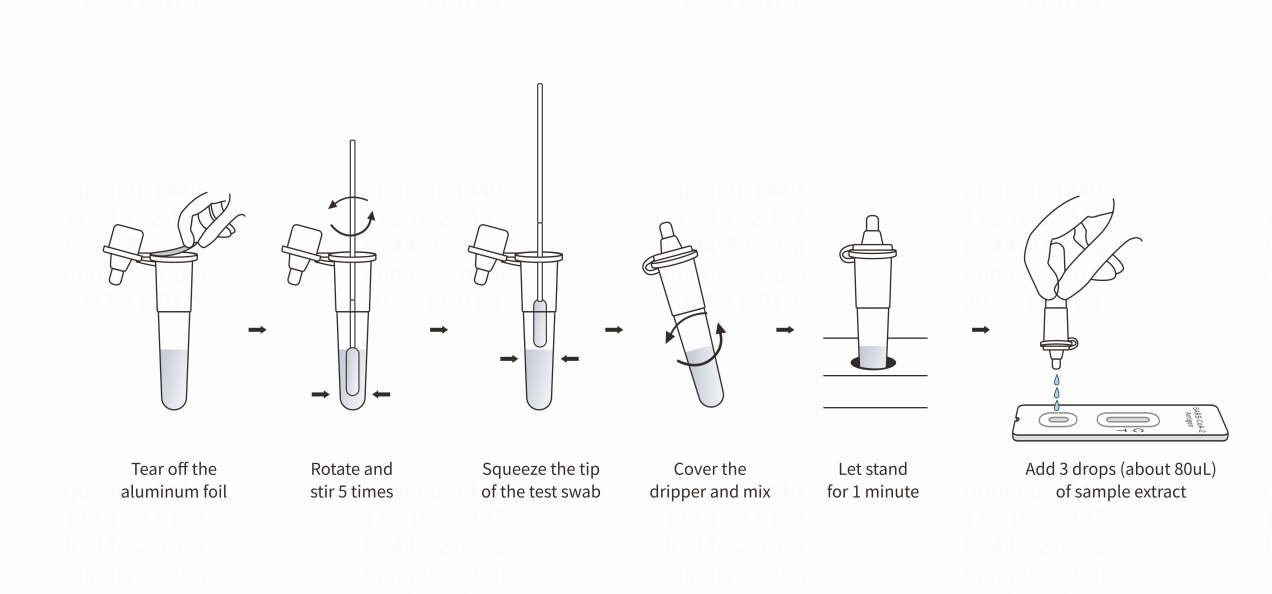
Result interpretation
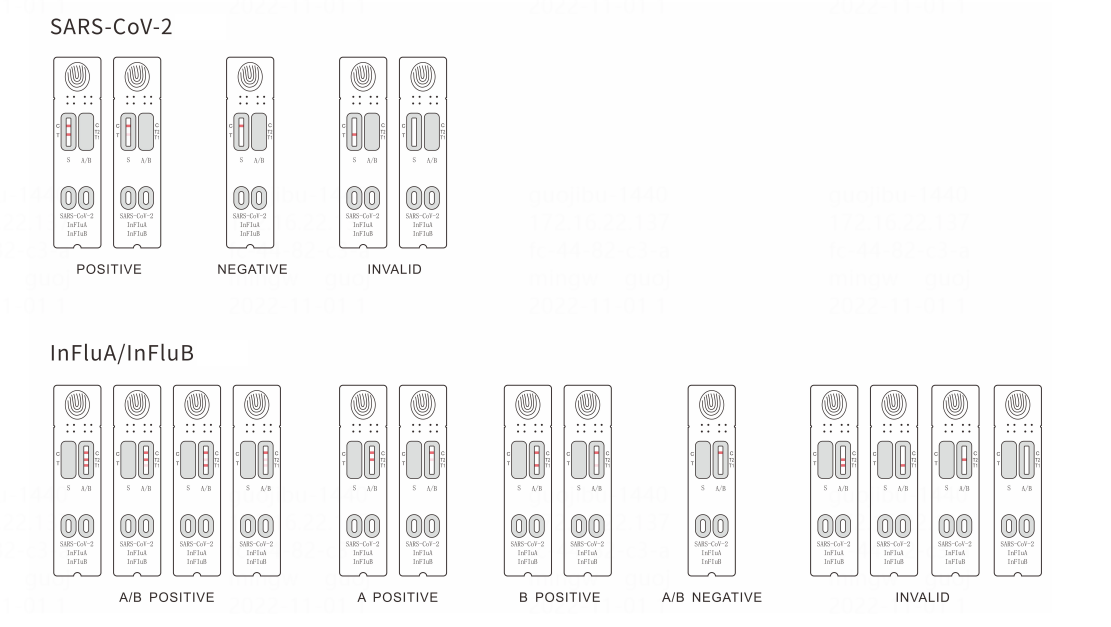
Parameters
Qualification CE Kit components
No. Product Name Spec. S3121E-25SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay)25 Test The kit components:
- SARS-CoV-2/InFluA/InFluB Antigen Test Cassette (individually in a foil pouch with desiccant)
- Sample Extraction Buffer
- Swab
