-

-

-


Archimed X4 Real-Time qPCR System
PCR InstrumentsFeatures
1. Innovative Optical Design(1) High sensitivity(2) Less cross-talk(3) Fast scan(4) No edge effects(5) Maintenance-free2. Outstanding Thermal Cycler(1) Compatible with 0.1ml or 0.2ml Low Profile 96-well plate, 8-strip tube, single tube(transparent, frosted, and milky white are applicable)(2) Precise temperature control of± 0.2 ℃ across the entire sample block(3) Innovative thermal block with max ramp rates of up to 3.6 ℃/s(4) Gradient function over 12 columns with a 36 ℃ spread3. Reliable Results with High-quality Data(1) Excellent reproducibility and 10-log dynamic range(2) Precise quantification with 1.33-fold discrimination(3) Broad linear dynamic range ensuring accurate quantification4. Intelligent Analysis for Multiple Applications(1) Accommodates user needs and different types of experiments with intuitive navigation and customizable settings.(2) Built-in data analysis modules with automatic baseline subtraction and threshold calculation for determining Ct values or possible standard curves and PCR efficiencies(3) Absolute quantification or relative quantification applicable(4) Includes analysis methods for probe-based allelic discrimniation and the use of a positive/negative analysis via the end-point detection of samplesSpecifications
Thermal Cycler Block capacity 96 Sample volume 10-50μl Heating/cooling method Peltier Max ramp rate 3.6℃/sec Temperature setting range 4-100℃ Heated lid Electronic automatic lid Temperature accuracy ±0.2℃ Temperature uniformity ±0.2℃ Gradient zone 12 columns Gradient range 1-36℃ Optical Detection Excitation source Long-life, high-performance LEDs Detector Highly sensitive MPPC with Fresnel lens Scanning principle Time-resolved scanning technology Detector position Top of the block Excitation/detection range 455-650nm/510-715nm Fluorescence channel 4 channels (Archimed X4) 6 channels (Archimed X6) Detection sensitivity 1 copy of the target sequence System sensitivity Detect differences as small as 1.33-fold in target quantities in singleplex reactions Dynamic range 10 orders of magnitude Dye compatibility FAM/SYBR Green, VIC/JOE/HEX/TET, NED/TAMRA/Cy3, JUN, ROX/Texas Red, Mustang Purple, Cy5/LIZ Data Analysis Modes Absolute quantification Relative quantification Endpoint qualitative analysis Melt curve analysis Protein Stability Screening Genotyping Data Export Customizable reports containing run settings, data graphs, and spreadsheets can be directly exported or saved as Excel, txt, PDFs -
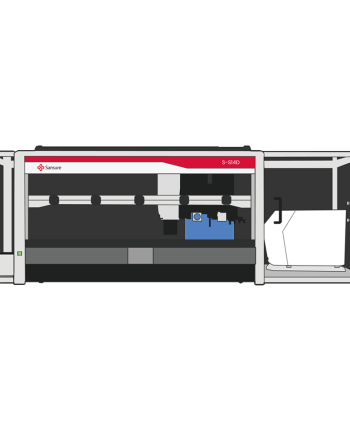

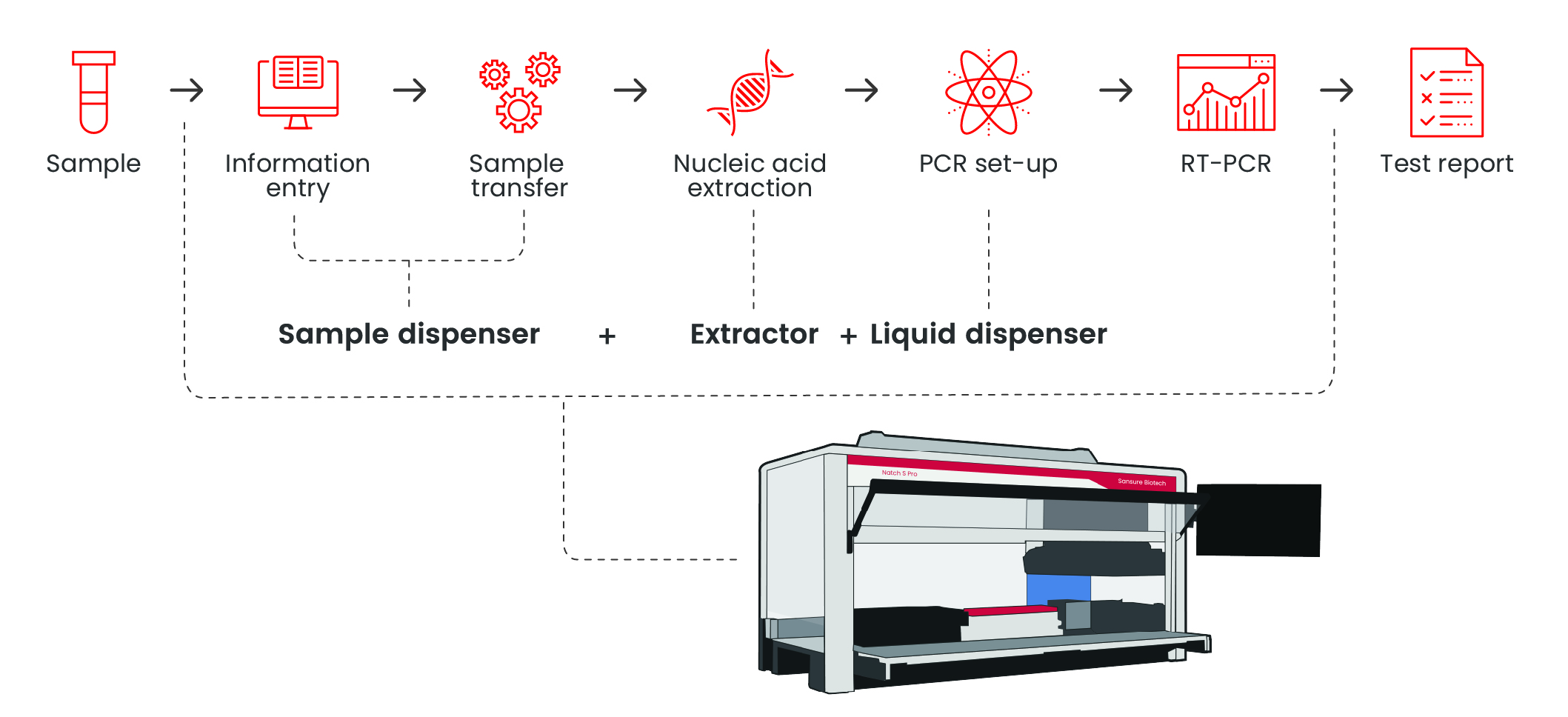
Natch S Pro Fully Automated Nucleic Acid Process System
Extraction InstrumentsWorkflow
Features
Integrated all-in-one platform for seamless workflow The unified system integrates sample preparation, nucleic acid extraction, and subsequent processing in a single device, eliminating manual transfers and minimizing cross-contamination risks. 1. CO-RE technology for precise pipetting
2. Low temperature preservation module to ensure enzymatic activity
Unique Compression-induced O-Ring Expansion (CO-RE) technology ensures precise tip at attachment and positioning.
3. Independent waste liquid channel to prevent countertop contamination
A low temperature module is equipped to prevent the reduction of enzyme activity
4. 96 HEAD, Greatly shorten nucleic acid extraction time
The waste liquid channel is designed to work separately from the workstation, ensuring the cleanliness of the laboratory table
5. One-click automatic operation, no need to be on duty throughout the process
Full board extraction time is reduced to 2hours Detection efficiency increased to 3.5h to complete 96 tests 
Interface operation is simplified, and the whole process of nucleic acid extraction can be automatically completed with one click Specifications
Instrument Model S-S14D Sample Type Serum, plasma, Whole blood Technology Platforms Advanced magnetic beads technology Sample Throughput 576 tests/batch Detection Time Pooling test: 576 tests/4.5 hours; Individual test: 96 tests/3.5 hours Sample Tube Spec. Compatible with original sample tubes of all sizes Pipetting Range 0.5-1000μL Pipetting Performance Pipetting volume 10μL, CV≤5%; 100μL, CV≤2%; 1000μL, CV≤1.5% Pipetting Channel 8 independent channels, with dual liquid level detection, clot detection, air tightness detection, tip detection, dynamic positioning and other functions. Liquid Level Detection Dual detection technology of pressure and capacitance can identify and record abnormal suction and discharge process (empty suction, missing suction, less suction, clot blockage) Extraction Principle Pipetting, side magnetism Magnetic Field Control Permanent magnet mode, three-dimensional surround magnetic attraction technology Tips Loading and Uploading CO-RE technology automatic tip loading and uploading Temperature Control Range Low temperature module: 0-15℃ adjustable Heating module: 5-105℃ adjustable Anti-contamination System UV lamp function, independent waste liquid channel Barcode Scanning Sample automatic scanning and sampling system (supports multiple barcodes), scanning time ≤ 1s Software System Window 7, Window 10 (recommended) Interface Type USB interface, RS232C serial port, support LIS system Operating Environment Temperature: 15-30 ℃ Humidity: 15%-85% (indoor, no condensation) Weight and Size About 220Kg, 2619mm(L)*903mm(H)*1043.5mm(W) Power Supply Voltage: 115VAC/230VAC, ±10% Frequency: 50/60Hz Certification NMPA -
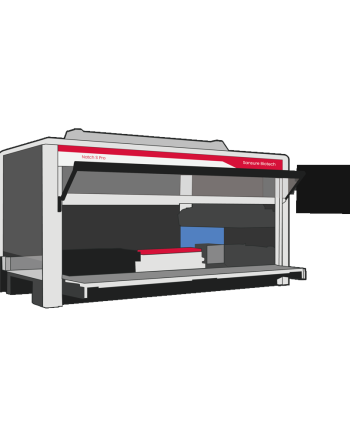

Natch S Pro Fully Automated Nucleic Acid Extraction System
Extraction InstrumentsWorkflow
Features
1. CO-RE technology for precise pipetting
2. Low temperature preservation module to ensure enzymatic activity
Unique Compression-induced O-Ring Expansion (CO-RE) technology ensures precise tip at attachment and positioning.
3. Independent waste liquid channel to prevent countertop contamination
A low temperature module is equipped to prevent the reduction of enzyme activity
4. 96 HEAD, Greatly shorten nucleic acid extraction time
The waste liquid channel is designed to work separately from the workstation, ensuring the cleanliness of the laboratory table
5. One-click automatic operation, no need to be on duty throughout the process
Full board extraction time is reduced to 2hours Detection efficiency increased to 3.5h to complete 96 tests 
Interface operation is simplified, and the whole process of nucleic acid extraction can be automatically completed with one click Specifications
Instrument Model S-S14A Sample Type Serum, plasma Technology Platforms Advanced magnetic beads technology Sample Throughput 576 tests/batch (pooling test); 96 tests/batch (individual test) Extraction Time 576 tests ≤ 3 hours; 96 tests ≤ 2.5 hours Sample Tube Spec. Compatible with original sample tubes of all sizes Pipetting Range 0.5-1000μL Pipetting Performance Pipetting volume 10μL, CV≤5%; 100μL, CV≤2%; 1000μL, CV≤1.5% Pipetting Channel 8 independent channels, with dual liquid level detection, clot detection, air tightness detection, tip detection, dynamic positioning and other functions. Liquid Level Detection Dual detection technology of pressure and capacitance can identify and record abnormal suction and discharge process (empty suction, missing suction, less suction, clot blockage) Extraction Principle Pipetting, side magnetism Magnetic Field Control Permanent magnet mode, three-dimensional surround magnetic attraction technology Tips Loading and Uploading CO-RE technology automatic tip loading and uploading Temperature Control Range Low temperature module: 0-15℃ adjustable Heating module: 5-105℃ adjustable Anti-contamination System UV lamp function, independent waste liquid channel Barcode Scanning Sample automatic scanning and sampling system (supports multiple barcodes), scanning time ≤ 1s Software System Window 7, Window 10 (recommended) Interface Type USB interface, RS232C serial port, support LIS system Operating Environment Temperature: 15-30 ℃ Humidity: 15%-85% (indoor, no condensation) Weight and Size About 150Kg, 1664mm(L)*903mm(H)*795mm(W) Power Supply Voltage: 115VAC/230VAC, ±10% Frequency: 50/60Hz Certification CE, NMPA -


S3214E B19 – Human Parvovirus B19 DNA Nucleic Acid Diagnostic Kit
Other InfectionsBrief
The Human Parvovirus B19 DNA Diagnostic Kit (PCR-Fluorescence Probing) is a real-time PCR test intended for the qualitative detection of nucleic acid from Human Parvovirus B19 in plasma, serum from individuals who are suspected of B19 infection.Parameters
Product features Parameter Specimen Type Plasma, serum Extraction Platform Advanced magnetic beads technology Extraction ReagentS1006EPCR Instrument MA6000, SLAN-96P, QuantStudio 5, ABI7500, Roche cobas 480 Sensitivity 400 copies/mL Spec. 24T, 48T Qualification CE -


S3179E HAV – Hepatitis A Virus RNA Nucleic Acid Diagnostic Kit
Other InfectionsBrief
The Hepatitis A Virus RNA Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) is a Real-time PCR test intended for the qualitative detection of nucleic acid from HAV in plasma and faeces.
Parameters
Product features Parameter Specimen Type Plasma Extraction Platform Advanced magnetic beads technology Extraction ReagentS1006EPCR Instrument MA6000, SLAN-96P, QuantStudio 5, ABI7500, Roche cobas 480, CFX96 Sensitivity 1000 copies/mL Spec. 24T Qualification CE -


SureXler 48 Real-Time PCR Instrument
PCR InstrumentsBrief
SureXler, the avant-garde leader in real-time fluorescent quantitative PCR analysis, is designed for on-the-go testing. Its six independent modules with 8-channel system offer seamless multiplex testing, simplifying even the most complex procedures into effortless tasks. With a sleek and portable design that fits easily into your backpack, you are empowered to conduct molecular detection with unmatched ease and precision. Elevate your testing experience to a new level of convenience and efficiency today.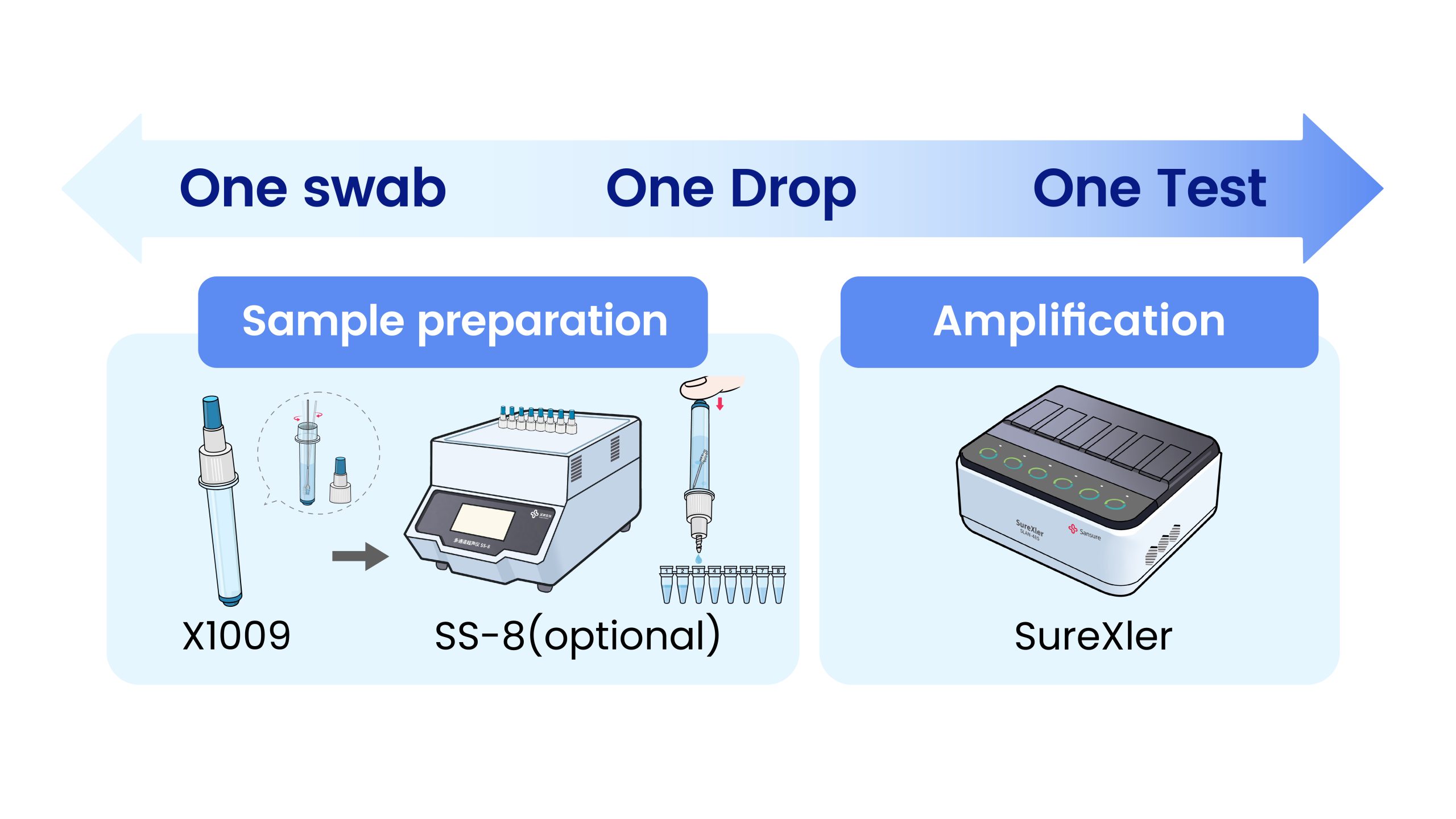
Manual Operation < 1 min, Results in 38 mins
Revolutionary temperature control technology 1. Amplification takes only 38 minutes, approximately half the time of conventional PCR instruments. 2. It enables the rapid and accurate detection of gene expression variations as minimal as 1.5-fold. Flexible and independent modules
1. Each module is equipped with integrated temperature control system and heat lid, allowing for immediate testing upon arrival.
2. It serves as a gradient fluorescence PCR system and can simultaneously set 6 temperature and time gradients to compare amplification effects and identify the optimal reaction conditions, aiding in reagent development.
Flexible and independent modules
1. Each module is equipped with integrated temperature control system and heat lid, allowing for immediate testing upon arrival.
2. It serves as a gradient fluorescence PCR system and can simultaneously set 6 temperature and time gradients to compare amplification effects and identify the optimal reaction conditions, aiding in reagent development.
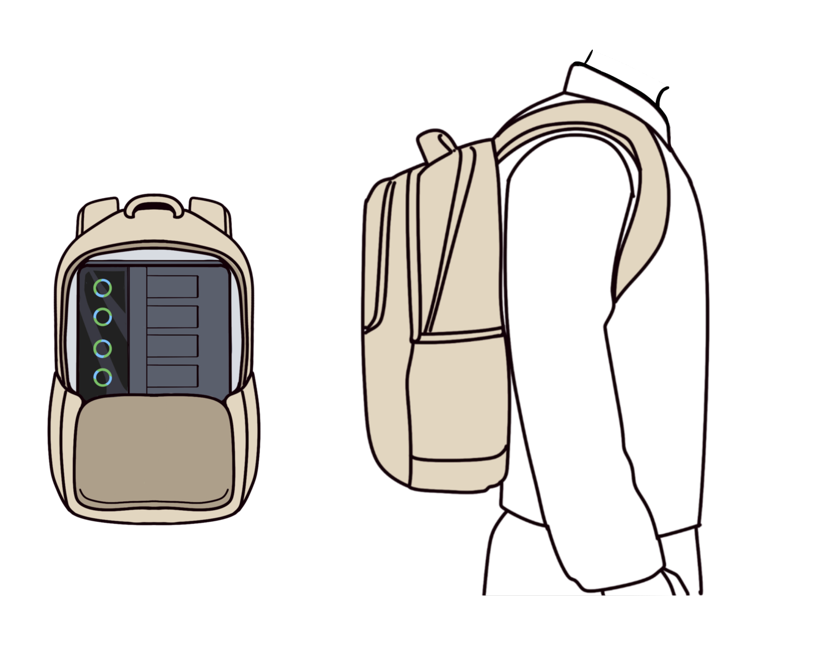 Smart Portable Design
1. Weighing under 10 kg, the instrument is one-third the weight of conventional thermal cyclers and can be carried in a backpack for molecular detection.
2. The instrument is operational without calibration after relocation.
3. Compatible with outdoor mobile power supplies for mobile detection.
Smart Portable Design
1. Weighing under 10 kg, the instrument is one-third the weight of conventional thermal cyclers and can be carried in a backpack for molecular detection.
2. The instrument is operational without calibration after relocation.
3. Compatible with outdoor mobile power supplies for mobile detection.
 Interoperable Open Platform
Compatible with various reagents, the device allows for customized experiments.
Interoperable Open Platform
Compatible with various reagents, the device allows for customized experiments.
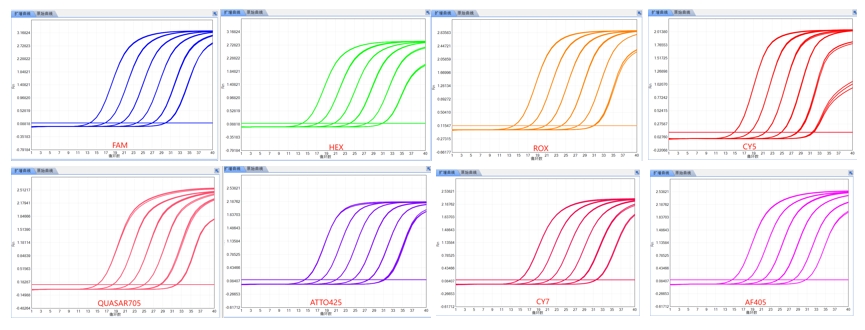 Eight-color fluorescence detection system
Detects up to 8 targets per well, enhancing efficiency.
Eight-color fluorescence detection system
Detects up to 8 targets per well, enhancing efficiency.
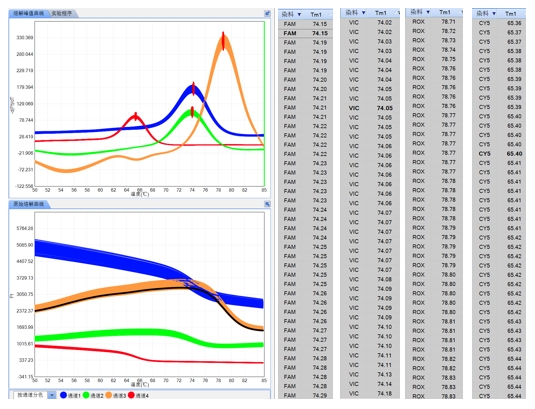 Robust Software System
The software offers various analysis modes, including qualitative/absolute quantification, melt curve analysis, endpoint genotyping, HRM analysis, isothermal amplification, etc.
Robust Software System
The software offers various analysis modes, including qualitative/absolute quantification, melt curve analysis, endpoint genotyping, HRM analysis, isothermal amplification, etc.
Specifications
Performance
Thermal SystemSample Capacity48 Well (6×8×0.2ml)Parallel Experiment6 reaction modules run up to 6 different experiments at the same time Consumables0.2ml PCR tubes, 4 strip tubesLight SourceHigh power Led (Maintenance Free)DetectorHigh sensitivity photoelectric sensorDynamic Range100 - 1010Sensitivity1 copyQuantitative RepeatabilityCV<1.00%CorrelationIrI≥0.9990DNA concentration Differentiation1.5 fold (Difference between 1000 and 1500 copy)Sample Volume15μL - 100μLProbe/DyeChannel 1: FAM, SYBR-Green Channel 2:VIC, HEX, JOE, TET Channel 3: ROX, Texas Red Channel 4: CY 5 Channel 5: QUASAR705, CY5.5 Channel 6: ATTO425 Channel 7: CY7 Channel 8: AF405 *Channel 7 and Channel 8 can be customized according to user needs.
Working ConditionControl ModeFast/StandardTemperature Indication Error±0.1℃ Temperature Uniformity±0.1℃ Temperature Control Range4℃- 99℃Heating Rate Avg8℃/SecMaximum Heating Rate10℃/SecCooling Rate Avg6℃/SecMaximum Cooling Rate8℃/SecHot-lid30℃ - 120℃(Default 105℃, Adjustable) Auto Hot-lid Operating SystemWindows 7/8/10/11SoftwarePackaged project managementPower600WPower Input100 - 240VAC 50/60HzSize343mm×295mm×157mmWeight9kgInterfaceUSB -


SNMC0021IR HPV G14 – High-risk Human Papillomavirus DNA (14 Genotypes) Diagnostic Kit
Reproductive Tract InfectionsBrief
This High-risk HPV DNA (Genotype) Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (type 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) present in exfoliated cells from females’ cervix. The test results can be used for identification of HPV genotypes.Parameters
Product features Parameter Specimen Type Exfoliated cells from females’ cervix Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 Internal Control β--globin gene PCR Instrument SLAN-96P, ABI 7500, Roche LC 480 and QuantStudioTM 5 , Stratagene Mx3000P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 24T -

iPonatic III Pro – Portable Molecular Diagnostic System
mPOCTBrief
To provide time-saving, labor-saving and safe solutions for efficient nucleic acid analysis is always the intention of research and development for Sansure iPonatic series product. The iPonatic III Pro combines the core technical advantages of the previous generations of iPonatic series products, integrates better fluid controlling, magnetic beads method for nucleic acid extraction and PCR process, to avoid the traditional PCR experimental process time-consuming, complex operation and other drawbacks. By truly realizing the "sample in, result out" fully automated gene detection, and with Sansure’s flexible and extended testing menu, iPonatic III Pro is the ultimate choice for efficient molecular diagnostics.Features
- Simplicity: Fully automated DNA/RNA extraction and detection to minimize manual labor
- Precision: Features fast PCR with real-time data analysis for reliable results
- Scalability: Manages up to 8 devices with iScreen, meeting diverse testing demands
- Speed: Delivers results within approximately 1 hour, ensuring rapid TAT time
- Compatibility: Processes various pathogens across different Viral Transport Media
- Intelligence: Offers wireless LIS and IoT connectivity for advanced disease surveillance
Parameters
Model S-Q37A (with ultrasonic function) S-Q37B (without ultrasonic function) Dimension About 400mm * 141mm * 401mm (L*W*H) WeightAbout 9.9kgFunctions Nucleic acid extraction, amplification & detection, data analysis Sample process Liquid transfer, heating and ultrasonic funciton (S-Q37A only) for DNA purification Analysis Support melting curve analysis Channels FAM, VIC, ROX, CY5 Maximum heating rate 10°C/sec Maximum cooling rate 3°C/sec Temperature accuracy 0.5°C Display Built-in 7-inch high-definition touch screen Communication USB2.0, RJ45, Type-C, Wi-Fi, Bluetooth, LIS_LH7 Input voltage 100~240VAC Power frequency 50/60Hz Rated power 140VA Operating temperature 10°C~30°C Operating humidity 20%RH~85%RH, non-condensing Barometric pressure 85.0kPa~106.0kPa Altitude Less than 3,000 m Transportation and storage Environment temperature range: -20°C~55°C Relative humidity range: ≤85%RH, non-condensing Test menu
Respiratory:- Six RP (Influenza A virus, Influenza B virus, Respiratory syncytial virus, Adenovirus, Human rhinovirus and Mycoplasma pneumonia)
- 6LRP (Klebsiella pneumoniae, Streptococcus pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa, Legionella pneumophila and Staphylococcus aureus)
- SARS-CoV-2/Flu/RSV (SARS-CoV-2, Influenza virus A/B (Flu) and Respiratory syncytial virus (RSV))
- COVID (ORF1ab and N genes of novel coronavirus (2019-nCoV))
- SARS-CoV-2/ FluA/B (SARS-CoV-2, Influenza A and Influenza B)
- MP (Mycoplasma pneumoniae)
- MERS (HCoV-MERS)
- TB and RIF (Mycobacterium tuberculosis and Rifampicin resistance mutations)
- HPV 13+2 (Human papillomavirus (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68))
- MG/MH/TV (Mycoplasma genitalium, Mycoplasma hominis, and Trichomonas vaginalis)
- EV/EV71/CA16 (Enterovirus, Coxsackievirus A16 and Enterovirus 71)
- GBS (Group B streptococcus)
- DENV/ZIKV/CHIKV (Dengue virus (DENV), Zika virus (ZIKV), and Chikungunya virus (CHIKV) RNA)
Use scenarios
Hospital, Nursing Home, Airport, Cruise, CDC, Clinic, Lab, Pharmacy, etc.
*All use scenarios must comply with local regulations.Product video
https://youtu.be/oGEpTLWrCIU?si=WUtry-NhRyFGeSup -


S3055E BP – Bordetella Pertussis DNA Diagnostic Kit
Respiratory Tract InfectionsBrief
Pertussis, also known as whooping cough, is a highly contagious respiratory infection caused by the bacterium Bordetella pertussis. Pertussis spreads easily from person to person mainly through droplets produced by coughing or sneezing. The disease is most dangerous in infants, and is a significant cause of disease and death in this age group.
Sansure kit is used to detect Bordetella pertussis DNA present in the nasopharyngeal swab specimens by applying PCR fluorescence probing technique. The detection result can be used as an aid in the diagnosis of bordetella pertussis.
Parameters
Product features Parameter Specimen Types Nasopharyngeal swabTechnical PlatformFast release technologyAdvanced magnetic beads technologyAnti-contamination systemUNG enzyme + dUTP systemInternal ControlInternal control plasmidPCR InstrumentsMx3000P; ABI 7500; MA-6000; S-Q31A/S-Q31B; S-Q36ASensitivity200 copies/mLObtained CertificatesNMPA, CE-IVDD etc. -


Natch 16S Nucleic Acid Extraction System
Extraction InstrumentsSpecifications
ModelNatch 16SSample throughput1-16Extraction technology Magnetic bead technologyPurification differences between wellsCV≤5%Mixing modeTip comb moving up and down to mix, 10 different mixing modesExtraction time10-60 minutes/batchAnti-contaminationBuilt-in UV lamp and air filtration systemOperation modeTouch controlProgram managementCreate, copy, delete, import, export and editDisplay7-inch touch screenInterfaceUSBPower supplyInput: AC 100V-240V, 50-60Hz, 120VASize≤200mm×300mm×300mm (L×D×H) Net weight≤8Kg Extraction kit components
No. Spec. Product Name S10016E 48T/kit Nucleic Acid Extraction-Purification Kit (Magnetic beads method) Available projects
Sexually Transmitted Diseases : CT, NG, UU, HSV-2, HSV I/2 Women's Health : hrHPV and hrHPV genotyping Respiratory Tract Disease : 6RP, TB, MP, RSV, ADV, BP Gastrointestinal Diseases : HFMD Prenatal and Postnatal care : CMV, EBV Public Health : SARS-CoV-2, Influenza A/B, HINI, H7N9 Customerized Projects -


S3174E HCoV-MERS – Human Coronavirus (MERS) Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
The Human Coronavirus (MERS) is generally detectable in respiratory samples during the acute phase of infection. The Human Coronavirus (MERS) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) can qualitatively detect HCoV-MERS in sputum, alveolar lavage fluid, and throat swabs, the test results can be used to assist in the diagnosis of patients infected with HCoV-MERS, providing a molecular diagnostic basis for coronavirus MERS infection. The test results of this kit are for clinical reference only, and should not be used as the sole criterion for clinical diagnosis. It is recommended to conduct a comprehensive analysis of the condition based on the patient's clinical manifestations and other laboratory tests.
Parameters
Product features Parameter Covering Genes MERS-CoV Orf1bSpecimen TypesSputum, alveolar lavage fluid, throat swabTechnical PlatformOne-tube fast release technologyAdvanced magnetic beads technologyInternal Control GeneRNase PCompatible InstrumentsABI 7500, Stratagene Mx3000P, SLAN®-96P, MA-6000, iPonatic S-Q36A/S-Q31A/S-Q31BSensitivity500 copies/mLQualificationCE -

S3104E 2019-nCoV (FDA EUA)Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing)
2019-nCoV (FDA EUA)For in vitro diagnostic use only.
For emergency use only.
For Prescription Use only.
Rx only.Brief
The Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from SARSCoV-2 in nasopharyngeal swabs, oropharyngeal (throat) swabs, anterior nasal swabs, midturbinate swabs, nasal washes and nasal aspirates from individuals who are suspected of COVID-19 by their healthcare provider. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet requirements to perform high complexity tests.
Results are for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA is generally detectable in respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2 RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
Performance
- One-tube/fast release technology
- Simple operation process, less specialist training
- Room temperature lysis, less contamination
- Sampling types: nasopharyngeal swabs, oropharyngeal (throat) swabs, anterior nasal swabs, midturbinate swabs, nasal washes and nasal spirates
- Internal control: human housekeeping gene RNase P
Parameters
NotesProduct features Parameters Specimen Type Nasopharyngeal swabs, oropharyngeal (throat) swabs, anterior nasal swabs, midturbinate swabs, nasal washes and nasal spirates Extraction Platform Sample Release Reagent Qiagen QIAamp Viral RNA Mini Kit Target GenesSARS-CoV-2 ORF1ab, N geneInternal ControlRnase P genePCR InstrumentABI 7500 Real-Time PCR System LoD200 copies/mLSpec.24T, 48TQualificationFDA EUA- This test has not been FDA cleared or approved;
- This test has been authorized by FDA under an EUA for use by authorized laboratories;
- This test has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens; and
- This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb- 3(b)(1), unless the authorization is terminated or revoked sooner.
-

S3353E FluA/Flu B – Influenza A/B Virus Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Influenza Virus is a kind of RNA virus in the Orthomyxoviridae family which leading to human and animal influenza. It causes acute upper respiratory tract infection, spreads rapidly through the air and has periodic pandemics around the world. Human influenza virus are influenza pathogens which can be classified into three types, namely A, B and C. Among them, influenza A is the most harmful, while influenza B and influenza C have weak pathogenicity and are not easy to mutate.
The diagnostic Kit is intended for detection of the Influenza A and Influenza B in oropharyngeal swab from individuals. The test results can be used for the auxiliary diagnosis of respiratory Influenza A/B Virus infection and provide molecular diagnostic basis for Influenza A/B Virus infection.
Parameters
Product features Parameter Covering pathogensInfluenza A and Influenza BSpecimen TypesOropharyngeal swabTechnical PlatformOne-tube fast release technologyAdvanced magnetic beads technologyCompatible InstrumentABI 7500; MA-6000; SLAN®-96P; QuantGene 9600; iPonatic S-Q31A/S-Q31BSensitivity200 copies/mLSpec.48T, 24-PQualificationCE -


S3363E-12-P TB and RFP – Mycobacterium Tuberculosis Nucleic Acid and Rifampicin Resistance Fluorescence Diagnostic Kit
Respiratory Tract InfectionsBrief
Mycobacterium tuberculosis (M. tuberculosis) is the pathogen causing tuberculosis, which can invade all organs of the whole body, and pulmonary tuberculosis caused by pulmonary involvement is the most common. Early diagnosis and treatment are crucial measures to effectively control the spread of tuberculosis.
Due to the abuse of antibiotics or the insufficient course of drugs of patients, the sensitivity of patients to drugs weakens or even disappears, resulting in the decreasing or ineffective effect of drugs on pulmonary tuberculosis. According to the types of anti-tuberculosis drugs, drug-resistant tuberculosis can be divided into monoresistance pulmonary tuberculosis, polyresistance pulmonary tuberculosis, multidrug resistance pulmonary tuberculosis and extensively drug-resistant pulmonary tuberculosis. Rifampicin is one of the first-line drugs for the treatment of pulmonary tuberculosis.
The Mycobacterium Tuberculosis and Rifampicin Resistance Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) is a real-time polymerase chain reaction test kit intended for the qualitative detection of the nucleic acid of mycobacterium tuberculosis and rifampicin resistance mutations in human sputum samples. The test results can be used to assist in the diagnosis of TB patients and patients with an increased risk of RFP drug-resistant TB, providing a molecular diagnosis basis for infected patients.
Parameters
The kit is registered in Indonesia.Product features Parameter Specimen Type Sputum Technical PlatformOne-tube fast release technologyPCR InstrumentsiPonatic Ⅲ (S-Q36A)Internal ControlRNase PLimit of detectionMycobacterium tuberculosis 1,000 Bacteria/mL;Rifampicin resistance 10,000 Bacteria/mL -


Sample Storage Reagent for SARS-CoV-2
Sample Storage ReagentBrief
The Sample Storage Reagent is intended for preservation and transportation of cells from human body.
The Sample Storage Reagent can protect the stability of virus and intracellular nucleic acid in clinical samples in a short term and is beneficial to the transport of clinical samples.
Kit Components
Item No.Main IngredientsX1002E0.9% normal saline, Rnasin and etc.X1003ESodium chloride, Rnasin and Guanidine Thiocyanate etc. X1004ETritonTM X-100, Tween-20 and ProClinTM 300 etc.Order Information
No.Product NameCapacitySpec.X1002ESample Storage Reagent1mL48T,96T/Kit2mL24T/Kit3mL24T,48T/Kit6mL24T,48T/KitX1003ESample Release Reagent1mL48T,96T/Kit2mL24T/Kit3mL24T,48T/Kit6mL24T,48T/KitX1004ESample Storage Reagent1mL48T,96T/Kit2mL24T/Kit3mL24T,48T/Kit6mL24T,48T/Kit -


S3108E HPV G23 – Human Papillomavirus DNA (23 genotypes) Diagnostic Kit
HPV InfectionsBrief
Human Papillomavirus DNA (23 genotypes) Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (Type 6, 11, 16, 18, 26, 31, 33, 35, 39, 42, 43, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 81, 82) present in exfoliated cells from females’ cervix. The test results can be used for identification of HPV genotypes.
Parameters
Product features Parameter Specimen Type Exfoliated cells from females’ cervixTechnical Platform One-tube fast release technology Advanced magnetic beads technology Detection types Type 6, 11, 16, 18, 26, 31, 33, 35, 39, 42, 43, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 81, 82Internal Control β--globin gene PCR Instrument Stratagene ABI7500, Life Technologies QuantStudioTM 5 and SLAN-96PAmplification Time 70 min Sensitivity 400 copies/mL Spec. 24T/48TQualification CE Order Information
Ref. No.Product NameSpec.S3108EHuman Papillomavirus DNA (23 genotypes) Diagnostic Kit (PCR-Fluorescence Probing)24T, 48T/KitS1013ESample Release Reagent48T/KitS10016ENucleic Acid Extraction-Purification Kit24T, 48T, 96T/Kit -


iPonatic III – Portable Molecular Workstation
mPOCTBrief
iPonatic III - Portable Molecular Workstation is a new member of Sansure's iPonatic series, optimizing molecular diagnostics with its advanced capabilities. With its cutting-edge technology, iPonatic III ushers in a new "digital and intelligent" era of molecular diagnostics, empowering healthcare professionals and providing rapid and accurate results.Features
- Fully automated rapid testing process and “sample-in-result-out” system make test results available in 15 - 45 minutes.
- Pre-packaged kits significantly shorten the hands-on time and reduce the chance of contamination.
- Rich and extendable testing menu meets the testing demands of different pathogens from every scenario.
- Through innovative wireless connection modules, large smart screen, and flexible combination abilities, samples can be tested anytime upon arrival.
- Only 7.2 kilograms (15.84 Pounds)
- Benefiting from state-of-the-art “SanUI” interactive system and HD smart touch screen, the test results will be displayed directly.
- Top international industrial design standard embodies Sansure’s distinctive design concepts.
Parameters
Model S-Q36ADimension 391 mm×140 mm×368 mm (L×W×H) WeightAbout 7.2kgChannelsFAM, VIC/HEX, ROX/Texas Red, CY5Duration15 - 45min (SARS-CoV-2)LOD 200 copies/mL (SARS-CoV-2)Maximum heating rate≥10℃/secMaximum cooling rate≥3℃/secTemperature accuracy±0.5°CFunctionsNucleic acid extraction, amplification detection, data analysisDisplayBuilt-in 7-inch high-definition touch screen, 12.1-inch smart screen (optional)Interfaces/communicationUSB2.0, RJ45, Type-C, WI-FI, Bluetooth, LIS_LH7 Input voltag100-240 VACPower frequency50/60HzRated power160VATemperatureOperating conditions:10°C- 30°CTransportation and storage: -40°C- 55°CHumidityOperating conditions: 30% - 80%, non-condensingTransportation and storage: ≤ 93%, non-condensingBarometric pressure85.0kPa - 106.0k PaAltitudeLess than 3,000 mQualificationCETest menu
RTI: SARS-CoV-2 (ORF1ab, N gene) SARS-CoV-2 (ORF1ab, N gene, E gene) SARS-CoV-2/Flu A/Flu B SARS-CoV-2/Flu/RSV Six Respiratory Pathogens (Flu A/Flu B/RSV/AdV/HRV/MP) Acinetobacter baumannii and Canidia albican (AB/CA) Flu A/Flu B Mycoplasma Pneumoniae (MP) Mycobacterium Tuberculosis (TB) Bordetella Pertussis (BP) Respiratory Syncytial Virus (RSV) Streptococcus Pneumoniae (SP) Carbapenemase Gene (KPC) MERS Legionella pneumophila (Lp) Adenovirus (AdV) STI & HPV: HPV 13+2 (Identifies HPV 16 and HPV 18, reports 13 other high risk types in pooled results) HPV 15 HR (Reports 15 high risk HPV types in pooled results) HPV 16&18 HPV 6&11 HSV-2 HSV-1&2 Mycoplasma Genitalium/Mycoplasma Hominis/Trichomonas Vaginalis (MG/MH/TV) Neisseria Gonorrhoeae (NG) Ureaplasma Urealyticum (UU) Mycoplasma Genitalium (MG) Chlamydia Trachomatis/Ureaplasma Urealyticum/Neisseria Gonorrhoeae(CT/UU/NG) Other infections: Epstein-Barr Virus (EBV) Group B Streptococcus (GBS) Toxigenic Clostridium difficile (CD) * Monkeypox Virus (MPXV) *Use scenarios
Medical laboratories, emergency rooms, fever clinics, remote areas, CDCs, airports, customs, etc.
*All use scenarios must comply with local regulations. -

S3046E HIV-1 – Human Immunodeficiency Virus Type 1 RNA Quantitative Fluorescence Diagnostic Kit
Blood-borne Infections (BBIs)Brief
The human immunodeficiency virus (HIV) is the causative agent for the worldwide AIDS epidemic, and it has taken nearly 33 million lives worldwide. Sansure's HIV-1 RNA Quantitative Fluorescence Diagnostic Kit is intended for quantitative detection of the HIV-1 Virus RNA in human EDTA plasma specimens. Sansure's HIV-1 RNA kit received CE certificate, supporting the company to provide more quality products and services for international customers, and to help prevent and control AIDS worldwide.Performance
Patented Modified-Capture Probe Assay Using exclusive modified super-paramagnetic nano-beads to absorb DNA/RNA in the sample; Heating free: Innovative lysis solution, no heating, less aerosol contamination; Single wash step: Unique combination of inorganic and organic solutions for reduced handling steps and reduced HBV DNA loss. Dual Target LTR and GAG gene regions are selected as targets for HIV detection to avoid missed detection, which can improve detection efficiency. High Sensitivity 25 IU/mL, meeting HIV-1 infection clinic guidelines. Internal Control The HIV-1 RNA Kits uses Internal Control is the full name to whole-process the HIV-1 extraction and amplification process to avoid false negative results.Parameters
Product features Parameter Specimen Type Plasma Extraction Platform Advanced magnetic beads technology Genotype HIV-1 Group M,N,O Internal Control Pseudoviruses PCR Instrument SLAN-96P, ABI7500, Roche cobas 480 Sensitivity 25 IU/mL Linear range 50—1.0E+08 IU/mL Spec. 48T Qualification CE -

S3016E MP – Mycoplasma Pneumoniae DNA Fluorescence Diagnostic Kit
Respiratory Tract InfectionsBrief
Mycoplasma pneumoniae (MP) is a pathogenic microorganism between bacteria and virus. It is mainly transmitted through buccal and nasal mucus by the air causing respiratory diseases, with the highest incidence in children and adolescents. Respiratory infection has the manifestations of pharyngitis and bronchitis, with a few cases causing infection to the lung. Recently, incidence among infants and children is increasing, therefore, early diagnosis and treatment can decrease the exacerbation of acute pneumonia in children. The development of molecular biology also draws more attention to the fluorescence quantitative PCR technology for the detection of MP-DNA. This diagnostic kit is an in vitro nucleic acid amplification test for the detection of mycoplasma pneumoniae DNA in humansputum and throat swab. It is intended for use as an aid in the diagnosis of an MP infection and providing a molecular-diagnostics-based solution.Parameters
Product features Parameter Specimen Type Sputum and throat swab Extraction Platform One-tube fast release technology Internal Control cloning plasmid containing the target gene fragment PCR Instrument ABI 7500, SLAN-96P,MA-6000, Roche LightCycler 480, iPonatic S-Q31A&B,S-Q36A Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -


S3066E 6RP – Six Respiratory Pathogens Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Respiratory infections are classified into the upper respiratory tract infections and lower respiratory tract infections. It refer to the pathogens that infect the nose, throat, trachea, bronchi or lungs, which principally cause diseases of tissues and organs outside the respiratory tract, manifested by fever, sore throat, cough, headache and other symptoms. The respiratory tract pathogen has the characteristics of strong infectivity, rapid spread, short incubation period and acute onset, etc. which seriously harm human health. After respiratory infection, symptoms are mostly similar. Sansure six respiratory pathogens joint detection kits can help doctors make differential diagnosis, accurately detect the pathogens that cause symptoms, and formulate treatment plans.Performance
- High sensitivity: Super-cis-nanometer magnetic bead technology; can achieve 500copies/mL
- Accurate identification: One test presented six results ; accurate guidance for rational clinical drug use
- Whole-process monitoring: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) human housekeeping gene as internal standard; Monitor the whole process of sampling, nucleic acid extraction and amplification
Parameters
Product features Parameter Specimen Type Nasopharyngeal swabs Extraction Platform Advanced magnetic beads technology Internal Control lentivirus particles(GAPDH) PCR Instrument SLAN-96P, ABI7500, S-Q36A Sensitivity Influenza A virus: 2.0 TCID50/mL Influenza B virus: 2.0 TCID50/mL Respiratory syncytial virus: 500.0 copies/mL Adenovirus: 500.0 copies/mL Mycoplasma pneumonia: 500.0 copies/mL Human rhinovirus: 500.0 copies/mL Spec. 24T, 12P Qualification CE -

S3334E AdV – Adenovirus Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Acute infectious disease caused by adenovirus, easily affects the mucous membranes of the respiratory and digestive tracts, the conjunctiva of the eyes, the urinary tract and the lymph nodes. The main manifestation is an acute upper respiratory tract infection. The population is generally susceptible, mostly the children. Infants are susceptible to adenovirus pneumonia, which is severe and has a high mortality rate. The source of infection is the patient and the latent infected person. The virus is excreted from the respiratory tract and conjunctival secretions, feces and urine, and is transmitted by airborne droplets, close contact and the feces-oral route. The Adenovirus Nucleic Acid Diagnostic Kit is used for nucleic acid testing of adenovirus in patients suspected of being adenovirus infections (e.g., fever, cough, wheezing, dyspnea, bronchopneumonia, upper respiratory tract infections, lung infections, etc.) or related close contacts, and the results can be used to assist in the diagnosis of adenovirus infection and provide a molecular diagnostic basis for adenovirus infection.Features
- High sensitivity: Detection sensitivity reaches 200 copies/mL.
- Quick and easy: Perfectly match Sansure's one-tube fast Sample Release Reagent, easy to operate.
- IC monitoring: IC(Internal Control) monitoring test process to avoid false negative results.
Parameters
Items Parameter Specimen Type Throat swab Extraction Platform One-tube fast release technology Advanced magnetic beads technology Anti-contamination system UNG enzyme + dUTP system PCR Instrument ABI 7500; MA-6000; SLAN-96P; QuantStudio 5; iPonatic S-Q31A&B; S-Q36A Sensitivity 200 copies/mL Qualification NMPA, CE -


S3018E TB – Mycobacterium Tuberculosis DNA Fluorescence Diagnostic Kit
Respiratory Tract InfectionsBrief
Mycobacterium tuberculosis virus (TB) is a pathogenic bacterium that causes tuberculosis. It is likely to infect all human tissues and organs, especially the lungs to cause pulmonary tuberculosis. Early diagnosis and treatment are important for effective control of tuberculosis. In recent years, with the development of molecular biology, nucleic acid fluorescence quantitative PCR method based on the mycobacterium tuberculosis nucleic acid has drawn more and more attention from researchers.Parameters
Product features Parameter Specimen Type Human sputum Extraction Platform One-tube fast release technology Internal Control Cloning plasmid, without TB target sequence PCR Instrument ABI 7500, SLAN-96P,MA-6000, Stratagene Mx3000P, iPonatic S-Q31A&B,S-Q36A Sensitivity 1 bacterium/mL Spec. 48T, 12-P Qualification CE -


S3310E 6LRP – Six Respiratory Pathogens Multiplex Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Lower respiratory infections (LRP) remained the world’s most deadly communicable disease, ranked as the 4th leading cause of death. In 2019 it claimed 2.6 million lives. Diseases of the lower respiratory tract include acute tracheitis, bronchitis, pneumonia, chronic bronchitis, chronic obstructive pulmonary disease, bronchiectasis, etc. They are caused by microbial infections such as viruses, bacteria, mycoplasma, chlamydia and legionella. Bacteria are the main pathogens of lower respiratory tract infections, with a wide variety of pathogens and complex clinical presentations. Due to the long detection period and low positive detection rate of traditional pathogenic tests, over 62% of adults with community-acquired pneumonia have no pathogenic basis. Failure to identify the cause quickly can delay treatment, exacerbate the disease and lead to death, and increase the development of antibiotic resistance.Features
- Highly efficient identification and rapid diagnosis: Six common bacteria of lower respiratory tract infections can be detected in one tube; a batch of 94 sample tests can finish in 100 minutes.
- Accurate and reliable, high detection rate: sensitive and specific, unaffected by antibacterial drugs, full internal control monitoring to avoid false negatives, UDG enzyme + dUTP anti- contamination measures to reduce false positives.
- Easy to operate and adaptable: automatic instruments are available, and the results can be intelligently analyzed by conventional fluorescent PCR instruments to meet the needs of medical laboratories , clinical Institutions, emergency and primary care etc.
Parameters
Items Parameter Specimen Type Sputum Extraction Platform Advanced magnetic beads technology Internal Control Plasmid PCR Instrument Thermofisher QuantStudio™ 5 and SLAN-96P Sensitivity 15 CFU/mL (Streptococcus pneumoniae) 340 CFU/mL (Legionella pneumophila) 625 CFU/mL (Haemophilus influenzae) 675 CFU/mL (Pseudomonas aeruginosa) 900 CFU/mL (Klebsiella pneumoniae) 2875 CFU/mL (Staphylococcus aureus) Qualification NMPA, CE -

S3352E MPXV – Monkeypox virus Nucleic Acid Diagnostic Kit
Other InfectionsBrief
Monkeypox is a disease of global public health importance as it not only affects countries in west and central Africa, but the rest of the world. Human-to-human transmission can result from close contact with respiratory secretions, skin lesions of an infected person or recently contaminated objects. Transmission via droplet respiratory particles usually requires prolonged face-to-face contact, which puts health workers, household members and other close contacts of active cases at greater risk. WHO recommends polymerase chain reaction (PCR) is the preferred laboratory test given its accuracy and sensitivity.Performance
- Test time ≤ 30 min
- Sensitivity: 200 copies/mL
- Suitable for PCR and iPonatic
Parameters
Items Parameters Specimen Type Vesicles or pustules, nasopharyngeal swab, oropharyngeal swab, serum, whole blood Extraction Platform One-tube fast release technology Advanced magnetic beads technology Internal Control Human gene PCR Instrument ABI 7500, QuantStudio™ 5, LightCycler 480, MA-6000, SLAN-96P, QuantGene 9600, Portable Molecule Workstation S-Q31A&B, Portable Molecular Workstation S-Q36A Sensitivity 200 copies/mL Spec. 48 T, 12-P Qualification CE
