-

-

-


Archimed X4 Real-Time qPCR System
PCR InstrumentsFeatures
1. Innovative Optical Design(1) High sensitivity(2) Less cross-talk(3) Fast scan(4) No edge effects(5) Maintenance-free2. Outstanding Thermal Cycler(1) Compatible with 0.1ml or 0.2ml Low Profile 96-well plate, 8-strip tube, single tube(transparent, frosted, and milky white are applicable)(2) Precise temperature control of± 0.2 ℃ across the entire sample block(3) Innovative thermal block with max ramp rates of up to 3.6 ℃/s(4) Gradient function over 12 columns with a 36 ℃ spread3. Reliable Results with High-quality Data(1) Excellent reproducibility and 10-log dynamic range(2) Precise quantification with 1.33-fold discrimination(3) Broad linear dynamic range ensuring accurate quantification4. Intelligent Analysis for Multiple Applications(1) Accommodates user needs and different types of experiments with intuitive navigation and customizable settings.(2) Built-in data analysis modules with automatic baseline subtraction and threshold calculation for determining Ct values or possible standard curves and PCR efficiencies(3) Absolute quantification or relative quantification applicable(4) Includes analysis methods for probe-based allelic discrimniation and the use of a positive/negative analysis via the end-point detection of samplesSpecifications
Thermal Cycler Block capacity 96 Sample volume 10-50μl Heating/cooling method Peltier Max ramp rate 3.6℃/sec Temperature setting range 4-100℃ Heated lid Electronic automatic lid Temperature accuracy ±0.2℃ Temperature uniformity ±0.2℃ Gradient zone 12 columns Gradient range 1-36℃ Optical Detection Excitation source Long-life, high-performance LEDs Detector Highly sensitive MPPC with Fresnel lens Scanning principle Time-resolved scanning technology Detector position Top of the block Excitation/detection range 455-650nm/510-715nm Fluorescence channel 4 channels (Archimed X4) 6 channels (Archimed X6) Detection sensitivity 1 copy of the target sequence System sensitivity Detect differences as small as 1.33-fold in target quantities in singleplex reactions Dynamic range 10 orders of magnitude Dye compatibility FAM/SYBR Green, VIC/JOE/HEX/TET, NED/TAMRA/Cy3, JUN, ROX/Texas Red, Mustang Purple, Cy5/LIZ Data Analysis Modes Absolute quantification Relative quantification Endpoint qualitative analysis Melt curve analysis Protein Stability Screening Genotyping Data Export Customizable reports containing run settings, data graphs, and spreadsheets can be directly exported or saved as Excel, txt, PDFs -

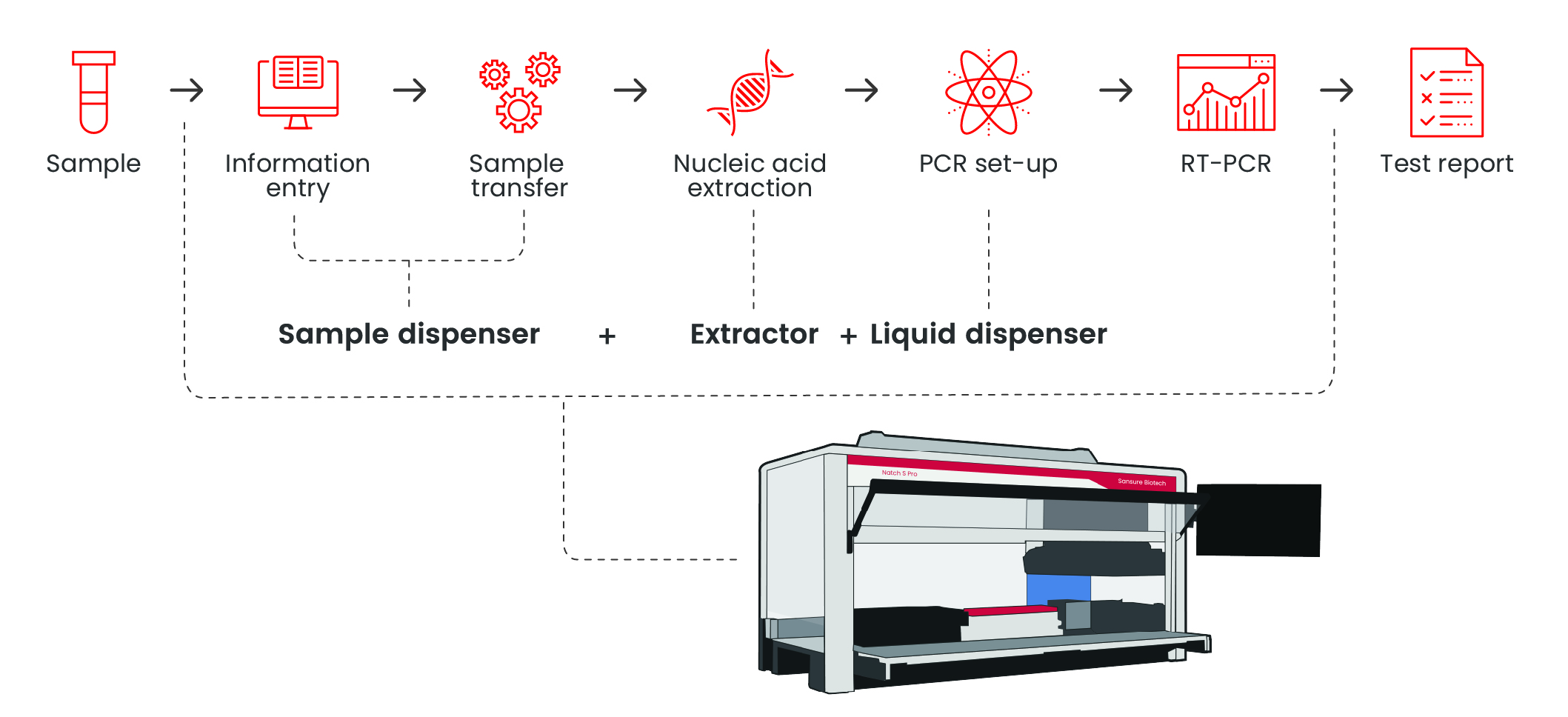
Natch S Pro Fully Automated Nucleic Acid Process System
Extraction InstrumentsWorkflow
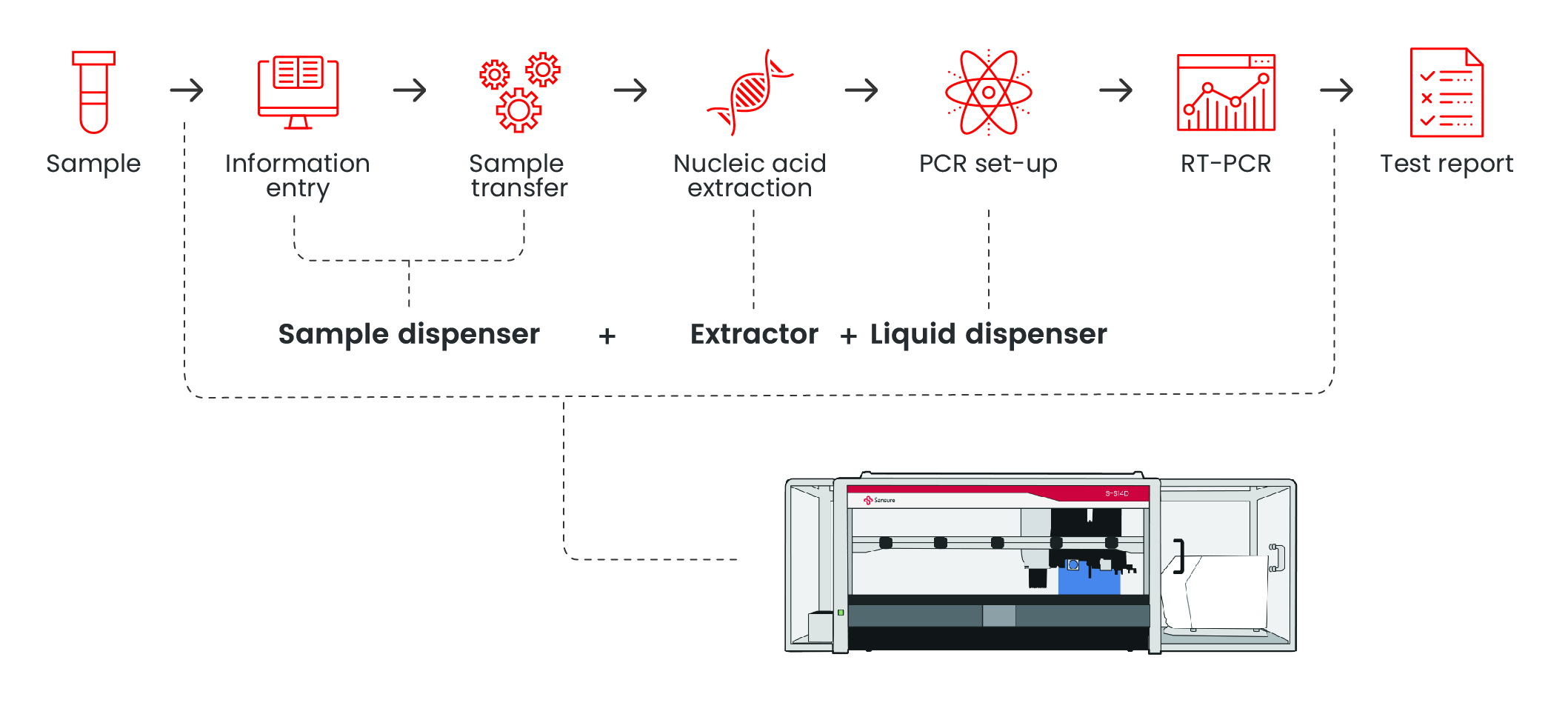
Features
Integrated all-in-one platform for seamless workflow The unified system integrates sample preparation, nucleic acid extraction, and subsequent processing in a single device, eliminating manual transfers and minimizing cross-contamination risks. 1. CO-RE technology for precise pipetting
2. Low temperature preservation module to ensure enzymatic activity
Unique Compression-induced O-Ring Expansion (CO-RE) technology ensures precise tip at attachment and positioning.
3. Independent waste liquid channel to prevent countertop contamination
A low temperature module is equipped to prevent the reduction of enzyme activity
4. 96 HEAD, Greatly shorten nucleic acid extraction time
The waste liquid channel is designed to work separately from the workstation, ensuring the cleanliness of the laboratory table
5. One-click automatic operation, no need to be on duty throughout the process
Full board extraction time is reduced to 2hours Detection efficiency increased to 3.5h to complete 96 tests 
Interface operation is simplified, and the whole process of nucleic acid extraction can be automatically completed with one click Specifications
Instrument Model S-S14D Sample Type Serum, plasma, Whole blood Technology Platforms Advanced magnetic beads technology Sample Throughput 576 tests/batch Detection Time Pooling test: 576 tests/4.5 hours; Individual test: 96 tests/3.5 hours Sample Tube Spec. Compatible with original sample tubes of all sizes Pipetting Range 0.5-1000μL Pipetting Performance Pipetting volume 10μL, CV≤5%; 100μL, CV≤2%; 1000μL, CV≤1.5% Pipetting Channel 8 independent channels, with dual liquid level detection, clot detection, air tightness detection, tip detection, dynamic positioning and other functions. Liquid Level Detection Dual detection technology of pressure and capacitance can identify and record abnormal suction and discharge process (empty suction, missing suction, less suction, clot blockage) Extraction Principle Pipetting, side magnetism Magnetic Field Control Permanent magnet mode, three-dimensional surround magnetic attraction technology Tips Loading and Uploading CO-RE technology automatic tip loading and uploading Temperature Control Range Low temperature module: 0-15℃ adjustable Heating module: 5-105℃ adjustable Anti-contamination System UV lamp function, independent waste liquid channel Barcode Scanning Sample automatic scanning and sampling system (supports multiple barcodes), scanning time ≤ 1s Software System Window 7, Window 10 (recommended) Interface Type USB interface, RS232C serial port, support LIS system Operating Environment Temperature: 15-30 ℃ Humidity: 15%-85% (indoor, no condensation) Weight and Size About 220Kg, 2619mm(L)*903mm(H)*1043.5mm(W) Power Supply Voltage: 115VAC/230VAC, ±10% Frequency: 50/60Hz Certification NMPA -

Natch S Pro Fully Automated Nucleic Acid Extraction System
Extraction InstrumentsWorkflow
Features
1. CO-RE technology for precise pipetting
2. Low temperature preservation module to ensure enzymatic activity
Unique Compression-induced O-Ring Expansion (CO-RE) technology ensures precise tip at attachment and positioning.
3. Independent waste liquid channel to prevent countertop contamination
A low temperature module is equipped to prevent the reduction of enzyme activity
4. 96 HEAD, Greatly shorten nucleic acid extraction time
The waste liquid channel is designed to work separately from the workstation, ensuring the cleanliness of the laboratory table
5. One-click automatic operation, no need to be on duty throughout the process
Full board extraction time is reduced to 2hours Detection efficiency increased to 3.5h to complete 96 tests 
Interface operation is simplified, and the whole process of nucleic acid extraction can be automatically completed with one click Specifications
Instrument Model S-S14A Sample Type Serum, plasma Technology Platforms Advanced magnetic beads technology Sample Throughput 576 tests/batch (pooling test); 96 tests/batch (individual test) Extraction Time 576 tests ≤ 3 hours; 96 tests ≤ 2.5 hours Sample Tube Spec. Compatible with original sample tubes of all sizes Pipetting Range 0.5-1000μL Pipetting Performance Pipetting volume 10μL, CV≤5%; 100μL, CV≤2%; 1000μL, CV≤1.5% Pipetting Channel 8 independent channels, with dual liquid level detection, clot detection, air tightness detection, tip detection, dynamic positioning and other functions. Liquid Level Detection Dual detection technology of pressure and capacitance can identify and record abnormal suction and discharge process (empty suction, missing suction, less suction, clot blockage) Extraction Principle Pipetting, side magnetism Magnetic Field Control Permanent magnet mode, three-dimensional surround magnetic attraction technology Tips Loading and Uploading CO-RE technology automatic tip loading and uploading Temperature Control Range Low temperature module: 0-15℃ adjustable Heating module: 5-105℃ adjustable Anti-contamination System UV lamp function, independent waste liquid channel Barcode Scanning Sample automatic scanning and sampling system (supports multiple barcodes), scanning time ≤ 1s Software System Window 7, Window 10 (recommended) Interface Type USB interface, RS232C serial port, support LIS system Operating Environment Temperature: 15-30 ℃ Humidity: 15%-85% (indoor, no condensation) Weight and Size About 150Kg, 1664mm(L)*903mm(H)*795mm(W) Power Supply Voltage: 115VAC/230VAC, ±10% Frequency: 50/60Hz Certification CE, NMPA -


S3214E B19 – Human Parvovirus B19 DNA Nucleic Acid Diagnostic Kit
Other InfectionsBrief
The Human Parvovirus B19 DNA Diagnostic Kit (PCR-Fluorescence Probing) is a real-time PCR test intended for the qualitative detection of nucleic acid from Human Parvovirus B19 in plasma, serum from individuals who are suspected of B19 infection.Parameters
Product features Parameter Specimen Type Plasma, serum Extraction Platform Advanced magnetic beads technology Extraction ReagentS1006EPCR Instrument MA6000, SLAN-96P, QuantStudio 5, ABI7500, Roche cobas 480 Sensitivity 400 copies/mL Spec. 24T, 48T Qualification CE -


S3179E HAV – Hepatitis A Virus RNA Nucleic Acid Diagnostic Kit
Other InfectionsBrief
The Hepatitis A Virus RNA Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) is a Real-time PCR test intended for the qualitative detection of nucleic acid from HAV in plasma and faeces.
Parameters
Product features Parameter Specimen Type Plasma Extraction Platform Advanced magnetic beads technology Extraction ReagentS1006EPCR Instrument MA6000, SLAN-96P, QuantStudio 5, ABI7500, Roche cobas 480, CFX96 Sensitivity 1000 copies/mL Spec. 24T Qualification CE -


SureXler 48 Real-Time PCR Instrument
PCR InstrumentsBrief
SureXler, the avant-garde leader in real-time fluorescent quantitative PCR analysis, is designed for on-the-go testing. Its six independent modules with 8-channel system offer seamless multiplex testing, simplifying even the most complex procedures into effortless tasks. With a sleek and portable design that fits easily into your backpack, you are empowered to conduct molecular detection with unmatched ease and precision. Elevate your testing experience to a new level of convenience and efficiency today.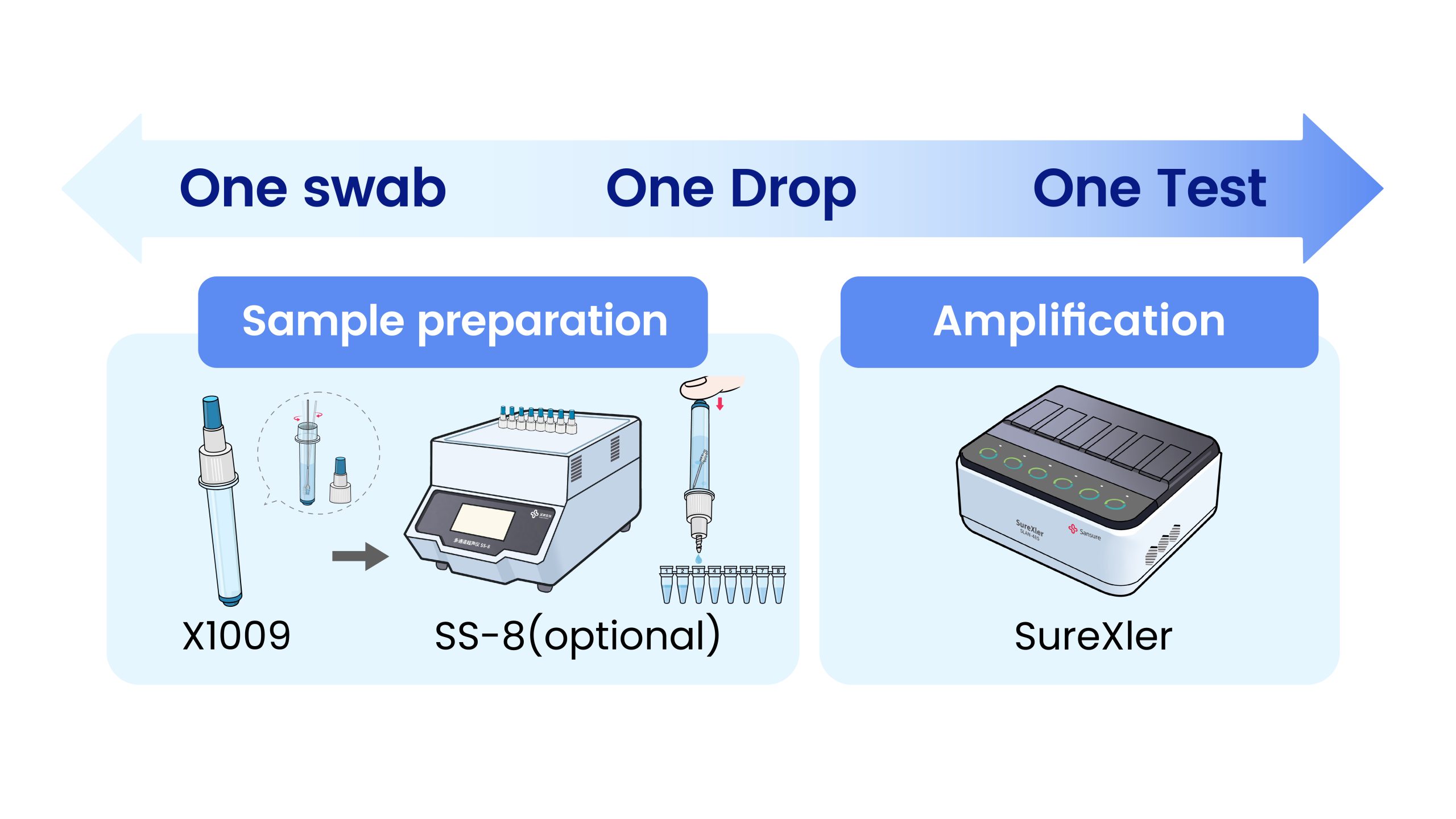
Manual Operation < 1 min, Results in 38 mins
Revolutionary temperature control technology 1. Amplification takes only 38 minutes, approximately half the time of conventional PCR instruments. 2. It enables the rapid and accurate detection of gene expression variations as minimal as 1.5-fold. Flexible and independent modules
1. Each module is equipped with integrated temperature control system and heat lid, allowing for immediate testing upon arrival.
2. It serves as a gradient fluorescence PCR system and can simultaneously set 6 temperature and time gradients to compare amplification effects and identify the optimal reaction conditions, aiding in reagent development.
Flexible and independent modules
1. Each module is equipped with integrated temperature control system and heat lid, allowing for immediate testing upon arrival.
2. It serves as a gradient fluorescence PCR system and can simultaneously set 6 temperature and time gradients to compare amplification effects and identify the optimal reaction conditions, aiding in reagent development.
 Smart Portable Design
1. Weighing under 10 kg, the instrument is one-third the weight of conventional thermal cyclers and can be carried in a backpack for molecular detection.
2. The instrument is operational without calibration after relocation.
3. Compatible with outdoor mobile power supplies for mobile detection.
Smart Portable Design
1. Weighing under 10 kg, the instrument is one-third the weight of conventional thermal cyclers and can be carried in a backpack for molecular detection.
2. The instrument is operational without calibration after relocation.
3. Compatible with outdoor mobile power supplies for mobile detection.
 Interoperable Open Platform
Compatible with various reagents, the device allows for customized experiments.
Interoperable Open Platform
Compatible with various reagents, the device allows for customized experiments.
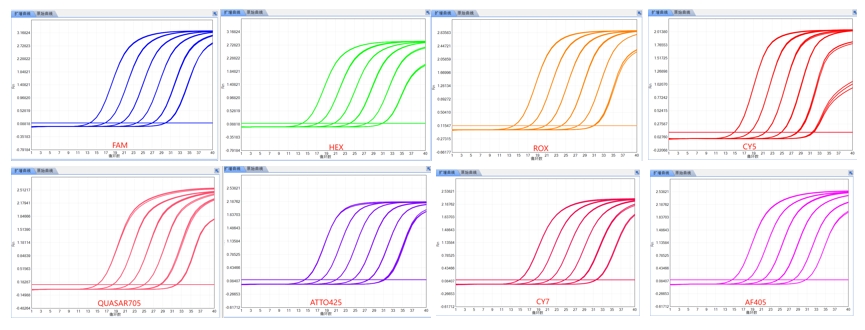 Eight-color fluorescence detection system
Detects up to 8 targets per well, enhancing efficiency.
Eight-color fluorescence detection system
Detects up to 8 targets per well, enhancing efficiency.
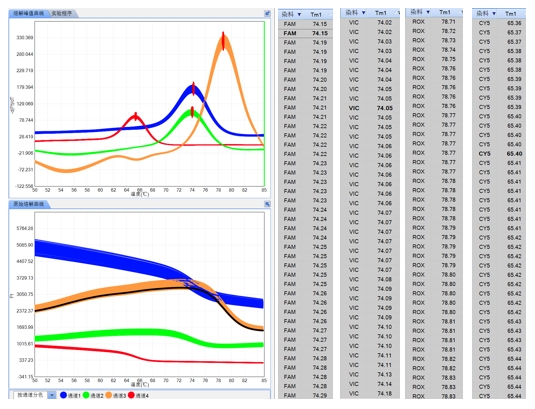 Robust Software System
The software offers various analysis modes, including qualitative/absolute quantification, melt curve analysis, endpoint genotyping, HRM analysis, isothermal amplification, etc.
Robust Software System
The software offers various analysis modes, including qualitative/absolute quantification, melt curve analysis, endpoint genotyping, HRM analysis, isothermal amplification, etc.
Specifications
Performance
Thermal SystemSample Capacity48 Well (6×8×0.2ml)Parallel Experiment6 reaction modules run up to 6 different experiments at the same time Consumables0.2ml PCR tubes, 4 strip tubesLight SourceHigh power Led (Maintenance Free)DetectorHigh sensitivity photoelectric sensorDynamic Range100 - 1010Sensitivity1 copyQuantitative RepeatabilityCV<1.00%CorrelationIrI≥0.9990DNA concentration Differentiation1.5 fold (Difference between 1000 and 1500 copy)Sample Volume15μL - 100μLProbe/DyeChannel 1: FAM, SYBR-Green Channel 2:VIC, HEX, JOE, TET Channel 3: ROX, Texas Red Channel 4: CY 5 Channel 5: QUASAR705, CY5.5 Channel 6: ATTO425 Channel 7: CY7 Channel 8: AF405 *Channel 7 and Channel 8 can be customized according to user needs.
Working ConditionControl ModeFast/StandardTemperature Indication Error±0.1℃ Temperature Uniformity±0.1℃ Temperature Control Range4℃- 99℃Heating Rate Avg8℃/SecMaximum Heating Rate10℃/SecCooling Rate Avg6℃/SecMaximum Cooling Rate8℃/SecHot-lid30℃ - 120℃(Default 105℃, Adjustable) Auto Hot-lid Operating SystemWindows 7/8/10/11SoftwarePackaged project managementPower600WPower Input100 - 240VAC 50/60HzSize343mm×295mm×157mmWeight9kgInterfaceUSB -


SNMC0021IR HPV G14 – High-risk Human Papillomavirus DNA (14 Genotypes) Diagnostic Kit
Reproductive Tract InfectionsBrief
This High-risk HPV DNA (Genotype) Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (type 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) present in exfoliated cells from females’ cervix. The test results can be used for identification of HPV genotypes.Parameters
Product features Parameter Specimen Type Exfoliated cells from females’ cervix Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 Internal Control β--globin gene PCR Instrument SLAN-96P, ABI 7500, Roche LC 480 and QuantStudioTM 5 , Stratagene Mx3000P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 24T -

iPonatic III Pro – Portable Molecular Diagnostic System
mPOCTBrief
To provide time-saving, labor-saving and safe solutions for efficient nucleic acid analysis is always the intention of research and development for Sansure iPonatic series product. The iPonatic III Pro combines the core technical advantages of the previous generations of iPonatic series products, integrates better fluid controlling, magnetic beads method for nucleic acid extraction and PCR process, to avoid the traditional PCR experimental process time-consuming, complex operation and other drawbacks. By truly realizing the "sample in, result out" fully automated gene detection, and with Sansure’s flexible and extended testing menu, iPonatic III Pro is the ultimate choice for efficient molecular diagnostics.Features
- Simplicity: Fully automated DNA/RNA extraction and detection to minimize manual labor
- Precision: Features fast PCR with real-time data analysis for reliable results
- Scalability: Manages up to 8 devices with iScreen, meeting diverse testing demands
- Speed: Delivers results within approximately 1 hour, ensuring rapid TAT time
- Compatibility: Processes various pathogens across different Viral Transport Media
- Intelligence: Offers wireless LIS and IoT connectivity for advanced disease surveillance
Parameters
Model S-Q37A (with ultrasonic function) S-Q37B (without ultrasonic function) Dimension About 400mm * 141mm * 401mm (L*W*H) WeightAbout 9.9kgFunctions Nucleic acid extraction, amplification & detection, data analysis Sample process Liquid transfer, heating and ultrasonic funciton (S-Q37A only) for DNA purification Analysis Support melting curve analysis Channels FAM, VIC, ROX, CY5 Maximum heating rate 10°C/sec Maximum cooling rate 3°C/sec Temperature accuracy 0.5°C Display Built-in 7-inch high-definition touch screen Communication USB2.0, RJ45, Type-C, Wi-Fi, Bluetooth, LIS_LH7 Input voltage 100~240VAC Power frequency 50/60Hz Rated power 140VA Operating temperature 10°C~30°C Operating humidity 20%RH~85%RH, non-condensing Barometric pressure 85.0kPa~106.0kPa Altitude Less than 3,000 m Transportation and storage Environment temperature range: -20°C~55°C Relative humidity range: ≤85%RH, non-condensing Test menu
Respiratory:- Six RP (Influenza A virus, Influenza B virus, Respiratory syncytial virus, Adenovirus, Human rhinovirus and Mycoplasma pneumonia)
- 6LRP (Klebsiella pneumoniae, Streptococcus pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa, Legionella pneumophila and Staphylococcus aureus)
- SARS-CoV-2/Flu/RSV (SARS-CoV-2, Influenza virus A/B (Flu) and Respiratory syncytial virus (RSV))
- COVID (ORF1ab and N genes of novel coronavirus (2019-nCoV))
- SARS-CoV-2/ FluA/B (SARS-CoV-2, Influenza A and Influenza B)
- MP (Mycoplasma pneumoniae)
- MERS (HCoV-MERS)
- TB and RIF (Mycobacterium tuberculosis and Rifampicin resistance mutations)
- HPV 13+2 (Human papillomavirus (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68))
- MG/MH/TV (Mycoplasma genitalium, Mycoplasma hominis, and Trichomonas vaginalis)
- EV/EV71/CA16 (Enterovirus, Coxsackievirus A16 and Enterovirus 71)
- GBS (Group B streptococcus)
- DENV/ZIKV/CHIKV (Dengue virus (DENV), Zika virus (ZIKV), and Chikungunya virus (CHIKV) RNA)
Use scenarios
Hospital, Nursing Home, Airport, Cruise, CDC, Clinic, Lab, Pharmacy, etc.
*All use scenarios must comply with local regulations.Product video
https://youtu.be/oGEpTLWrCIU?si=WUtry-NhRyFGeSup -


S3055E BP – Bordetella Pertussis DNA Diagnostic Kit
Respiratory Tract InfectionsBrief
Pertussis, also known as whooping cough, is a highly contagious respiratory infection caused by the bacterium Bordetella pertussis. Pertussis spreads easily from person to person mainly through droplets produced by coughing or sneezing. The disease is most dangerous in infants, and is a significant cause of disease and death in this age group.
Sansure kit is used to detect Bordetella pertussis DNA present in the nasopharyngeal swab specimens by applying PCR fluorescence probing technique. The detection result can be used as an aid in the diagnosis of bordetella pertussis.
Parameters
Product features Parameter Specimen Types Nasopharyngeal swabTechnical PlatformFast release technologyAdvanced magnetic beads technologyAnti-contamination systemUNG enzyme + dUTP systemInternal ControlInternal control plasmidPCR InstrumentsMx3000P; ABI 7500; MA-6000; S-Q31A/S-Q31B; S-Q36ASensitivity200 copies/mLObtained CertificatesNMPA, CE-IVDD etc. -


Natch 16S Nucleic Acid Extraction System
Extraction InstrumentsSpecifications
ModelNatch 16SSample throughput1-16Extraction technology Magnetic bead technologyPurification differences between wellsCV≤5%Mixing modeTip comb moving up and down to mix, 10 different mixing modesExtraction time10-60 minutes/batchAnti-contaminationBuilt-in UV lamp and air filtration systemOperation modeTouch controlProgram managementCreate, copy, delete, import, export and editDisplay7-inch touch screenInterfaceUSBPower supplyInput: AC 100V-240V, 50-60Hz, 120VASize≤200mm×300mm×300mm (L×D×H) Net weight≤8Kg Extraction kit components
No. Spec. Product Name S10016E 48T/kit Nucleic Acid Extraction-Purification Kit (Magnetic beads method) Available projects
Sexually Transmitted Diseases : CT, NG, UU, HSV-2, HSV I/2 Women's Health : hrHPV and hrHPV genotyping Respiratory Tract Disease : 6RP, TB, MP, RSV, ADV, BP Gastrointestinal Diseases : HFMD Prenatal and Postnatal care : CMV, EBV Public Health : SARS-CoV-2, Influenza A/B, HINI, H7N9 Customerized Projects -


S3174E HCoV-MERS – Human Coronavirus (MERS) Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
The Human Coronavirus (MERS) is generally detectable in respiratory samples during the acute phase of infection. The Human Coronavirus (MERS) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) can qualitatively detect HCoV-MERS in sputum, alveolar lavage fluid, and throat swabs, the test results can be used to assist in the diagnosis of patients infected with HCoV-MERS, providing a molecular diagnostic basis for coronavirus MERS infection. The test results of this kit are for clinical reference only, and should not be used as the sole criterion for clinical diagnosis. It is recommended to conduct a comprehensive analysis of the condition based on the patient's clinical manifestations and other laboratory tests.
Parameters
Product features Parameter Covering Genes MERS-CoV Orf1bSpecimen TypesSputum, alveolar lavage fluid, throat swabTechnical PlatformOne-tube fast release technologyAdvanced magnetic beads technologyInternal Control GeneRNase PCompatible InstrumentsABI 7500, Stratagene Mx3000P, SLAN®-96P, MA-6000, iPonatic S-Q36A/S-Q31A/S-Q31BSensitivity500 copies/mLQualificationCE -

S3104E 2019-nCoV (FDA EUA)Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing)
2019-nCoV (FDA EUA)For in vitro diagnostic use only.
For emergency use only.
For Prescription Use only.
Rx only.Brief
The Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from SARSCoV-2 in nasopharyngeal swabs, oropharyngeal (throat) swabs, anterior nasal swabs, midturbinate swabs, nasal washes and nasal aspirates from individuals who are suspected of COVID-19 by their healthcare provider. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet requirements to perform high complexity tests.
Results are for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA is generally detectable in respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2 RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
Performance
- One-tube/fast release technology
- Simple operation process, less specialist training
- Room temperature lysis, less contamination
- Sampling types: nasopharyngeal swabs, oropharyngeal (throat) swabs, anterior nasal swabs, midturbinate swabs, nasal washes and nasal spirates
- Internal control: human housekeeping gene RNase P
Parameters
NotesProduct features Parameters Specimen Type Nasopharyngeal swabs, oropharyngeal (throat) swabs, anterior nasal swabs, midturbinate swabs, nasal washes and nasal spirates Extraction Platform Sample Release Reagent Qiagen QIAamp Viral RNA Mini Kit Target GenesSARS-CoV-2 ORF1ab, N geneInternal ControlRnase P genePCR InstrumentABI 7500 Real-Time PCR System LoD200 copies/mLSpec.24T, 48TQualificationFDA EUA- This test has not been FDA cleared or approved;
- This test has been authorized by FDA under an EUA for use by authorized laboratories;
- This test has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens; and
- This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb- 3(b)(1), unless the authorization is terminated or revoked sooner.
-

S3353E FluA/Flu B – Influenza A/B Virus Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Influenza Virus is a kind of RNA virus in the Orthomyxoviridae family which leading to human and animal influenza. It causes acute upper respiratory tract infection, spreads rapidly through the air and has periodic pandemics around the world. Human influenza virus are influenza pathogens which can be classified into three types, namely A, B and C. Among them, influenza A is the most harmful, while influenza B and influenza C have weak pathogenicity and are not easy to mutate.
The diagnostic Kit is intended for detection of the Influenza A and Influenza B in oropharyngeal swab from individuals. The test results can be used for the auxiliary diagnosis of respiratory Influenza A/B Virus infection and provide molecular diagnostic basis for Influenza A/B Virus infection.
Parameters
Product features Parameter Covering pathogensInfluenza A and Influenza BSpecimen TypesOropharyngeal swabTechnical PlatformOne-tube fast release technologyAdvanced magnetic beads technologyCompatible InstrumentABI 7500; MA-6000; SLAN®-96P; QuantGene 9600; iPonatic S-Q31A/S-Q31BSensitivity200 copies/mLSpec.48T, 24-PQualificationCE -


S3363E-12-P TB and RFP – Mycobacterium Tuberculosis Nucleic Acid and Rifampicin Resistance Fluorescence Diagnostic Kit
Respiratory Tract InfectionsBrief
Mycobacterium tuberculosis (M. tuberculosis) is the pathogen causing tuberculosis, which can invade all organs of the whole body, and pulmonary tuberculosis caused by pulmonary involvement is the most common. Early diagnosis and treatment are crucial measures to effectively control the spread of tuberculosis.
Due to the abuse of antibiotics or the insufficient course of drugs of patients, the sensitivity of patients to drugs weakens or even disappears, resulting in the decreasing or ineffective effect of drugs on pulmonary tuberculosis. According to the types of anti-tuberculosis drugs, drug-resistant tuberculosis can be divided into monoresistance pulmonary tuberculosis, polyresistance pulmonary tuberculosis, multidrug resistance pulmonary tuberculosis and extensively drug-resistant pulmonary tuberculosis. Rifampicin is one of the first-line drugs for the treatment of pulmonary tuberculosis.
The Mycobacterium Tuberculosis and Rifampicin Resistance Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) is a real-time polymerase chain reaction test kit intended for the qualitative detection of the nucleic acid of mycobacterium tuberculosis and rifampicin resistance mutations in human sputum samples. The test results can be used to assist in the diagnosis of TB patients and patients with an increased risk of RFP drug-resistant TB, providing a molecular diagnosis basis for infected patients.
Parameters
The kit is registered in Indonesia.Product features Parameter Specimen Type Sputum Technical PlatformOne-tube fast release technologyPCR InstrumentsiPonatic Ⅲ (S-Q36A)Internal ControlRNase PLimit of detectionMycobacterium tuberculosis 1,000 Bacteria/mL;Rifampicin resistance 10,000 Bacteria/mL -


Sample Storage Reagent for SARS-CoV-2
Sample Storage ReagentBrief
The Sample Storage Reagent is intended for preservation and transportation of cells from human body.
The Sample Storage Reagent can protect the stability of virus and intracellular nucleic acid in clinical samples in a short term and is beneficial to the transport of clinical samples.
Kit Components
Item No.Main IngredientsX1002E0.9% normal saline, Rnasin and etc.X1003ESodium chloride, Rnasin and Guanidine Thiocyanate etc. X1004ETritonTM X-100, Tween-20 and ProClinTM 300 etc.Order Information
No.Product NameCapacitySpec.X1002ESample Storage Reagent1mL48T,96T/Kit2mL24T/Kit3mL24T,48T/Kit6mL24T,48T/KitX1003ESample Release Reagent1mL48T,96T/Kit2mL24T/Kit3mL24T,48T/Kit6mL24T,48T/KitX1004ESample Storage Reagent1mL48T,96T/Kit2mL24T/Kit3mL24T,48T/Kit6mL24T,48T/Kit -


S3108E HPV G23 – Human Papillomavirus DNA (23 genotypes) Diagnostic Kit
HPV InfectionsBrief
Human Papillomavirus DNA (23 genotypes) Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (Type 6, 11, 16, 18, 26, 31, 33, 35, 39, 42, 43, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 81, 82) present in exfoliated cells from females’ cervix. The test results can be used for identification of HPV genotypes.
Parameters
Product features Parameter Specimen Type Exfoliated cells from females’ cervixTechnical Platform One-tube fast release technology Advanced magnetic beads technology Detection types Type 6, 11, 16, 18, 26, 31, 33, 35, 39, 42, 43, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 81, 82Internal Control β--globin gene PCR Instrument Stratagene ABI7500, Life Technologies QuantStudioTM 5 and SLAN-96PAmplification Time 70 min Sensitivity 400 copies/mL Spec. 24T/48TQualification CE Order Information
Ref. No.Product NameSpec.S3108EHuman Papillomavirus DNA (23 genotypes) Diagnostic Kit (PCR-Fluorescence Probing)24T, 48T/KitS1013ESample Release Reagent48T/KitS10016ENucleic Acid Extraction-Purification Kit24T, 48T, 96T/Kit -


iPonatic III – Portable Molecular Workstation
mPOCTBrief
iPonatic III - Portable Molecular Workstation is a new member of Sansure's iPonatic series, optimizing molecular diagnostics with its advanced capabilities. With its cutting-edge technology, iPonatic III ushers in a new "digital and intelligent" era of molecular diagnostics, empowering healthcare professionals and providing rapid and accurate results.Features
- Fully automated rapid testing process and “sample-in-result-out” system make test results available in 15 - 45 minutes.
- Pre-packaged kits significantly shorten the hands-on time and reduce the chance of contamination.
- Rich and extendable testing menu meets the testing demands of different pathogens from every scenario.
- Through innovative wireless connection modules, large smart screen, and flexible combination abilities, samples can be tested anytime upon arrival.
- Only 7.2 kilograms (15.84 Pounds)
- Benefiting from state-of-the-art “SanUI” interactive system and HD smart touch screen, the test results will be displayed directly.
- Top international industrial design standard embodies Sansure’s distinctive design concepts.
Parameters
Model S-Q36ADimension 391 mm×140 mm×368 mm (L×W×H) WeightAbout 7.2kgChannelsFAM, VIC/HEX, ROX/Texas Red, CY5Duration15 - 45min (SARS-CoV-2)LOD 200 copies/mL (SARS-CoV-2)Maximum heating rate≥10℃/secMaximum cooling rate≥3℃/secTemperature accuracy±0.5°CFunctionsNucleic acid extraction, amplification detection, data analysisDisplayBuilt-in 7-inch high-definition touch screen, 12.1-inch smart screen (optional)Interfaces/communicationUSB2.0, RJ45, Type-C, WI-FI, Bluetooth, LIS_LH7 Input voltag100-240 VACPower frequency50/60HzRated power160VATemperatureOperating conditions:10°C- 30°CTransportation and storage: -40°C- 55°CHumidityOperating conditions: 30% - 80%, non-condensingTransportation and storage: ≤ 93%, non-condensingBarometric pressure85.0kPa - 106.0k PaAltitudeLess than 3,000 mQualificationCETest menu
RTI: SARS-CoV-2 (ORF1ab, N gene) SARS-CoV-2 (ORF1ab, N gene, E gene) SARS-CoV-2/Flu A/Flu B SARS-CoV-2/Flu/RSV Six Respiratory Pathogens (Flu A/Flu B/RSV/AdV/HRV/MP) Acinetobacter baumannii and Canidia albican (AB/CA) Flu A/Flu B Mycoplasma Pneumoniae (MP) Mycobacterium Tuberculosis (TB) Bordetella Pertussis (BP) Respiratory Syncytial Virus (RSV) Streptococcus Pneumoniae (SP) Carbapenemase Gene (KPC) MERS Legionella pneumophila (Lp) Adenovirus (AdV) STI & HPV: HPV 13+2 (Identifies HPV 16 and HPV 18, reports 13 other high risk types in pooled results) HPV 15 HR (Reports 15 high risk HPV types in pooled results) HPV 16&18 HPV 6&11 HSV-2 HSV-1&2 Mycoplasma Genitalium/Mycoplasma Hominis/Trichomonas Vaginalis (MG/MH/TV) Neisseria Gonorrhoeae (NG) Ureaplasma Urealyticum (UU) Mycoplasma Genitalium (MG) Chlamydia Trachomatis/Ureaplasma Urealyticum/Neisseria Gonorrhoeae(CT/UU/NG) Other infections: Epstein-Barr Virus (EBV) Group B Streptococcus (GBS) Toxigenic Clostridium difficile (CD) * Monkeypox Virus (MPXV) *Use scenarios
Medical laboratories, emergency rooms, fever clinics, remote areas, CDCs, airports, customs, etc.
*All use scenarios must comply with local regulations. -

S3046E HIV-1 – Human Immunodeficiency Virus Type 1 RNA Quantitative Fluorescence Diagnostic Kit
Blood-borne Infections (BBIs)Brief
The human immunodeficiency virus (HIV) is the causative agent for the worldwide AIDS epidemic, and it has taken nearly 33 million lives worldwide. Sansure's HIV-1 RNA Quantitative Fluorescence Diagnostic Kit is intended for quantitative detection of the HIV-1 Virus RNA in human EDTA plasma specimens. Sansure's HIV-1 RNA kit received CE certificate, supporting the company to provide more quality products and services for international customers, and to help prevent and control AIDS worldwide.Performance
Patented Modified-Capture Probe Assay Using exclusive modified super-paramagnetic nano-beads to absorb DNA/RNA in the sample; Heating free: Innovative lysis solution, no heating, less aerosol contamination; Single wash step: Unique combination of inorganic and organic solutions for reduced handling steps and reduced HBV DNA loss. Dual Target LTR and GAG gene regions are selected as targets for HIV detection to avoid missed detection, which can improve detection efficiency. High Sensitivity 25 IU/mL, meeting HIV-1 infection clinic guidelines. Internal Control The HIV-1 RNA Kits uses Internal Control is the full name to whole-process the HIV-1 extraction and amplification process to avoid false negative results.Parameters
Product features Parameter Specimen Type Plasma Extraction Platform Advanced magnetic beads technology Genotype HIV-1 Group M,N,O Internal Control Pseudoviruses PCR Instrument SLAN-96P, ABI7500, Roche cobas 480 Sensitivity 25 IU/mL Linear range 50—1.0E+08 IU/mL Spec. 48T Qualification CE -

S3016E MP – Mycoplasma Pneumoniae DNA Fluorescence Diagnostic Kit
Respiratory Tract InfectionsBrief
Mycoplasma pneumoniae (MP) is a pathogenic microorganism between bacteria and virus. It is mainly transmitted through buccal and nasal mucus by the air causing respiratory diseases, with the highest incidence in children and adolescents. Respiratory infection has the manifestations of pharyngitis and bronchitis, with a few cases causing infection to the lung. Recently, incidence among infants and children is increasing, therefore, early diagnosis and treatment can decrease the exacerbation of acute pneumonia in children. The development of molecular biology also draws more attention to the fluorescence quantitative PCR technology for the detection of MP-DNA. This diagnostic kit is an in vitro nucleic acid amplification test for the detection of mycoplasma pneumoniae DNA in humansputum and throat swab. It is intended for use as an aid in the diagnosis of an MP infection and providing a molecular-diagnostics-based solution.Parameters
Product features Parameter Specimen Type Sputum and throat swab Extraction Platform One-tube fast release technology Internal Control cloning plasmid containing the target gene fragment PCR Instrument ABI 7500, SLAN-96P,MA-6000, Roche LightCycler 480, iPonatic S-Q31A&B,S-Q36A Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -


S3066E 6RP – Six Respiratory Pathogens Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Respiratory infections are classified into the upper respiratory tract infections and lower respiratory tract infections. It refer to the pathogens that infect the nose, throat, trachea, bronchi or lungs, which principally cause diseases of tissues and organs outside the respiratory tract, manifested by fever, sore throat, cough, headache and other symptoms. The respiratory tract pathogen has the characteristics of strong infectivity, rapid spread, short incubation period and acute onset, etc. which seriously harm human health. After respiratory infection, symptoms are mostly similar. Sansure six respiratory pathogens joint detection kits can help doctors make differential diagnosis, accurately detect the pathogens that cause symptoms, and formulate treatment plans.Performance
- High sensitivity: Super-cis-nanometer magnetic bead technology; can achieve 500copies/mL
- Accurate identification: One test presented six results ; accurate guidance for rational clinical drug use
- Whole-process monitoring: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) human housekeeping gene as internal standard; Monitor the whole process of sampling, nucleic acid extraction and amplification
Parameters
Product features Parameter Specimen Type Nasopharyngeal swabs Extraction Platform Advanced magnetic beads technology Internal Control lentivirus particles(GAPDH) PCR Instrument SLAN-96P, ABI7500, S-Q36A Sensitivity Influenza A virus: 2.0 TCID50/mL Influenza B virus: 2.0 TCID50/mL Respiratory syncytial virus: 500.0 copies/mL Adenovirus: 500.0 copies/mL Mycoplasma pneumonia: 500.0 copies/mL Human rhinovirus: 500.0 copies/mL Spec. 24T, 12P Qualification CE -

S3334E AdV – Adenovirus Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Acute infectious disease caused by adenovirus, easily affects the mucous membranes of the respiratory and digestive tracts, the conjunctiva of the eyes, the urinary tract and the lymph nodes. The main manifestation is an acute upper respiratory tract infection. The population is generally susceptible, mostly the children. Infants are susceptible to adenovirus pneumonia, which is severe and has a high mortality rate. The source of infection is the patient and the latent infected person. The virus is excreted from the respiratory tract and conjunctival secretions, feces and urine, and is transmitted by airborne droplets, close contact and the feces-oral route. The Adenovirus Nucleic Acid Diagnostic Kit is used for nucleic acid testing of adenovirus in patients suspected of being adenovirus infections (e.g., fever, cough, wheezing, dyspnea, bronchopneumonia, upper respiratory tract infections, lung infections, etc.) or related close contacts, and the results can be used to assist in the diagnosis of adenovirus infection and provide a molecular diagnostic basis for adenovirus infection.Features
- High sensitivity: Detection sensitivity reaches 200 copies/mL.
- Quick and easy: Perfectly match Sansure's one-tube fast Sample Release Reagent, easy to operate.
- IC monitoring: IC(Internal Control) monitoring test process to avoid false negative results.
Parameters
Items Parameter Specimen Type Throat swab Extraction Platform One-tube fast release technology Advanced magnetic beads technology Anti-contamination system UNG enzyme + dUTP system PCR Instrument ABI 7500; MA-6000; SLAN-96P; QuantStudio 5; iPonatic S-Q31A&B; S-Q36A Sensitivity 200 copies/mL Qualification NMPA, CE -


S3018E TB – Mycobacterium Tuberculosis DNA Fluorescence Diagnostic Kit
Respiratory Tract InfectionsBrief
Mycobacterium tuberculosis virus (TB) is a pathogenic bacterium that causes tuberculosis. It is likely to infect all human tissues and organs, especially the lungs to cause pulmonary tuberculosis. Early diagnosis and treatment are important for effective control of tuberculosis. In recent years, with the development of molecular biology, nucleic acid fluorescence quantitative PCR method based on the mycobacterium tuberculosis nucleic acid has drawn more and more attention from researchers.Parameters
Product features Parameter Specimen Type Human sputum Extraction Platform One-tube fast release technology Internal Control Cloning plasmid, without TB target sequence PCR Instrument ABI 7500, SLAN-96P,MA-6000, Stratagene Mx3000P, iPonatic S-Q31A&B,S-Q36A Sensitivity 1 bacterium/mL Spec. 48T, 12-P Qualification CE -


S3310E 6LRP – Six Respiratory Pathogens Multiplex Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Lower respiratory infections (LRP) remained the world’s most deadly communicable disease, ranked as the 4th leading cause of death. In 2019 it claimed 2.6 million lives. Diseases of the lower respiratory tract include acute tracheitis, bronchitis, pneumonia, chronic bronchitis, chronic obstructive pulmonary disease, bronchiectasis, etc. They are caused by microbial infections such as viruses, bacteria, mycoplasma, chlamydia and legionella. Bacteria are the main pathogens of lower respiratory tract infections, with a wide variety of pathogens and complex clinical presentations. Due to the long detection period and low positive detection rate of traditional pathogenic tests, over 62% of adults with community-acquired pneumonia have no pathogenic basis. Failure to identify the cause quickly can delay treatment, exacerbate the disease and lead to death, and increase the development of antibiotic resistance.Features
- Highly efficient identification and rapid diagnosis: Six common bacteria of lower respiratory tract infections can be detected in one tube; a batch of 94 sample tests can finish in 100 minutes.
- Accurate and reliable, high detection rate: sensitive and specific, unaffected by antibacterial drugs, full internal control monitoring to avoid false negatives, UDG enzyme + dUTP anti- contamination measures to reduce false positives.
- Easy to operate and adaptable: automatic instruments are available, and the results can be intelligently analyzed by conventional fluorescent PCR instruments to meet the needs of medical laboratories , clinical Institutions, emergency and primary care etc.
Parameters
Items Parameter Specimen Type Sputum Extraction Platform Advanced magnetic beads technology Internal Control Plasmid PCR Instrument Thermofisher QuantStudio™ 5 and SLAN-96P Sensitivity 15 CFU/mL (Streptococcus pneumoniae) 340 CFU/mL (Legionella pneumophila) 625 CFU/mL (Haemophilus influenzae) 675 CFU/mL (Pseudomonas aeruginosa) 900 CFU/mL (Klebsiella pneumoniae) 2875 CFU/mL (Staphylococcus aureus) Qualification NMPA, CE -

S3352E MPXV – Monkeypox virus Nucleic Acid Diagnostic Kit
Other InfectionsBrief
Monkeypox is a disease of global public health importance as it not only affects countries in west and central Africa, but the rest of the world. Human-to-human transmission can result from close contact with respiratory secretions, skin lesions of an infected person or recently contaminated objects. Transmission via droplet respiratory particles usually requires prolonged face-to-face contact, which puts health workers, household members and other close contacts of active cases at greater risk. WHO recommends polymerase chain reaction (PCR) is the preferred laboratory test given its accuracy and sensitivity.Performance
- Test time ≤ 30 min
- Sensitivity: 200 copies/mL
- Suitable for PCR and iPonatic
Parameters
Items Parameters Specimen Type Vesicles or pustules, nasopharyngeal swab, oropharyngeal swab, serum, whole blood Extraction Platform One-tube fast release technology Advanced magnetic beads technology Internal Control Human gene PCR Instrument ABI 7500, QuantStudio™ 5, LightCycler 480, MA-6000, SLAN-96P, QuantGene 9600, Portable Molecule Workstation S-Q31A&B, Portable Molecular Workstation S-Q36A Sensitivity 200 copies/mL Spec. 48 T, 12-P Qualification CE -

C004E BCI – Nucleic Acid Test Kit for HBV, HCV, HIV (Type1+2)
Blood-borne Infections (BBIs)Brief
Nucleic Acid Test Kit for HBV,HCV,HIV(Type1+2), is based on real-time fluorescence PCR technology and used for nucleic acid qualitative detection of HBV, HCV, HIV1+2 in plasma. This kit is intended for use as a blood donor screening test to detect HBV DNA, HCV RNA, HIV-1 RNA and HIV-2 RNA in pooled or individual sample from healthy blood donors, blood donors of various components (red blood cells, platelets and plasma) and other types of blood donors. All plasma to be tested can be screened as individual samples or tested in pools after mixing with each equal aliquots according to routine serological screening results HBV, HCV and HIV samples. For the pooled sample showed positive test results, carry out individual testing, the individual test result should be used as the final test result of this sample. The test results of this diagnostic kit can distinguish reactivity of between HBV, HCV and HIV.Advantages
High sensitivity- HBV 2.41 IU/mL
- HCV 12.38 IU/mL
- HIV-1 31.68 IU/mL
- HIV-2 48.34 IU/mL
- 576 tests/ 5h(pooling)
- 96 tests / 4.5h(single)
- HBV genotypes A-H
- HCV genotypes 1-6
- HIV-1 group M/N/O
- HIV-2
- Four tests for a tube of samples
- Direct discriminating positive
- Extraction and amplification process
- No need to be on duty
- One extractor + one amplifier
- Less consumable material consumption
Features
Sample preparation and extraction in one module Fully automatic sample preparation and nucleic acid extraction in one module to build up a integrated reaction system Flexible testing mode 6 pooling sample testing or individual donor testing (IDT) supported Advanced magnetic beads technology Nanometer-level beads enable beads-in-PCR amplification ensuring maximum nucleic acid template Patented magnetic beads lateral suction technology Thorough waste liquid removal allows minimal magnetic beads loss Sophisticated sample information processing Automatic recognition of the sample barcode & sample tracking and archiving table generation available High throughput 558/96 samples result output in one time Minimal system maintenance time Less than 20 minutes startup with fewer maintenance tasks Excellent accuracy A total number of 105,124 blood bags which confirmed as negative by serological testing were tested against some referential screening kits. 15 false-negative results in reference tests were found.Parameters
Qualification CE -
S3120E-H-SARS-CoV-2 Rapid Antigen Test for Self-testing
Respiratory Tract InfectionsBrief
The SARS-CoV-2 Rapid Antigen Test (Immunochromatography Assay) for self-testing is authorized for home use with self-collected nasal swab samples to directly detect antigen of SARS-CoV-2 virus. With the help of this kit, people without professional training can also easily acquire their COVID-19 test result within 15-20 minutes.Features
- Accurate Sensitivity 94.55%, Specificity 100%
- Convenient No instrument needed, easy to operate
- Fast Result available in only 15-20 min
- Flexible Get tested anytime when you are free
Instruction
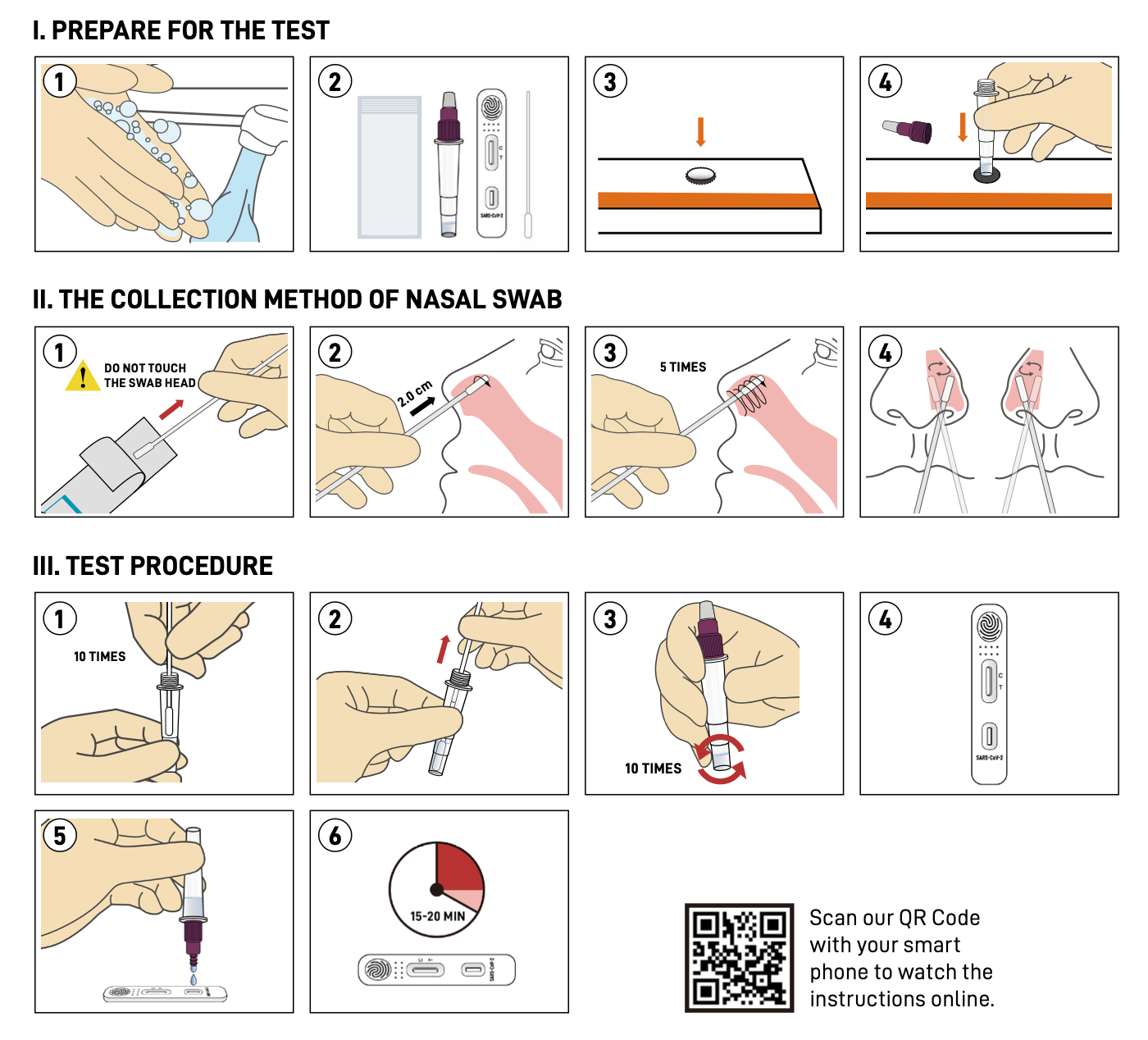
Result interpretation
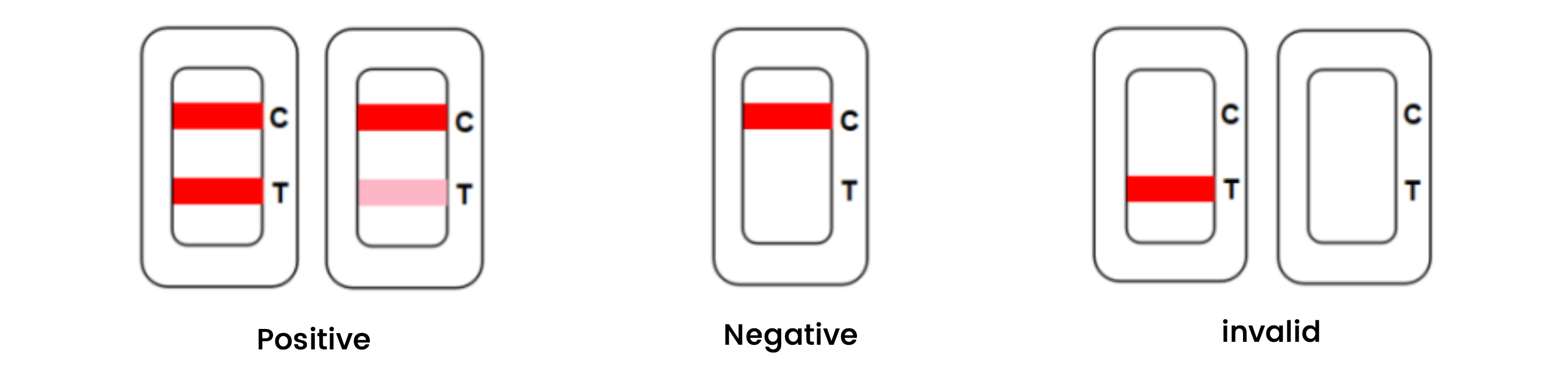
Parameters
Qualification CE Kit components
No. Product Name Spec. S3120E-1-H, S3120E-5-H SARS-CoV-2 Rapid Antigen Test (Immunochromatography Assay) 1 Test, 5 Tests The kit components:
- SARS-CoV-2-Antigen Test Cassette (with desiccant)
- SARS-CoV-2 Sample Extraction Buffer
- Swab
- Plastic Waste Bag
-

S3109E SARS-CoV-2 Rapid Antigen Test (Colloidal Gold Method)
Respiratory Tract InfectionsBrief
The SARS-CoV-2 Rapid Antigen Test (Colloidal Gold Method) is intended for the qualitative detection of the SARS-CoV-2 nucleocapsid protein in human nasopharyngeal or nasal swab sampled from individuals suspected of COVID-19.Parameters
Testing Time 10-15 minutes Sensitivity 98.4% Specificity 98.1% Qualification CE Kit components
No. Product Name Spec. S3109E-25 SARS-CoV-2 Rapid Antigen Test (Colloidal Gold Method) 25 tests -


iPonatic – Portable Molecule Workstation
mPOCT, PCR InstrumentsBrief
Innovations in molecular assays—especially on the point-of-care testing (POCT) front—have spread this testing from molecular diagnostics laboratories into clinical microbiology laboratories—and now into general laboratories and even clinics and exam rooms.[1] Access to sensitive and rapid infectious disease diagnostic assays is essential for accurate diagnosis, effective treatment, and timely infection control, making POCT vital to reducing TAT. With novel POCT on the horizon, future studies are warranted to determine cost savings, antimicrobial usage, TAT, patient impact, and how to best implement in non-microbiology clinical laboratories and clinics.[2] Sansure iPonatic (Portable Molecule Workstation) aims to innovate traditional diagnostic mode and assist precision diagnosis. It can provide quickly and convenient diagnosis experience for clinical emergency, health management, military safety, biological emergency and other applications.Core technologies
- Rapid nucleic acid lysis at room temperature within 1 minute
- Ultra fast amplification system in 8-45 minutes
- Integrated automatic data analysis software
- Results immediately printed by built-in printer
Performance
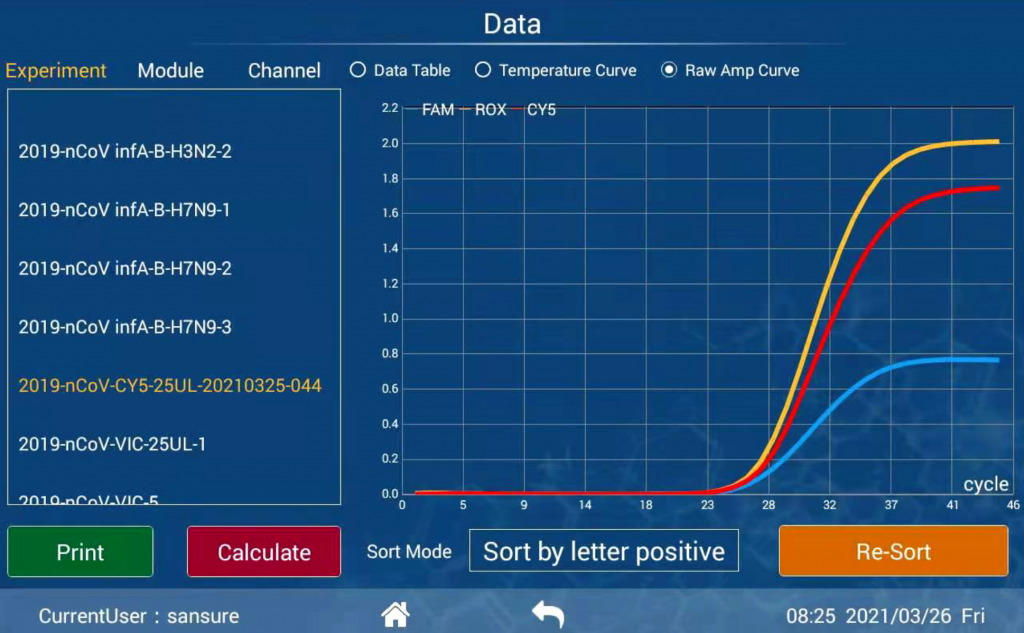
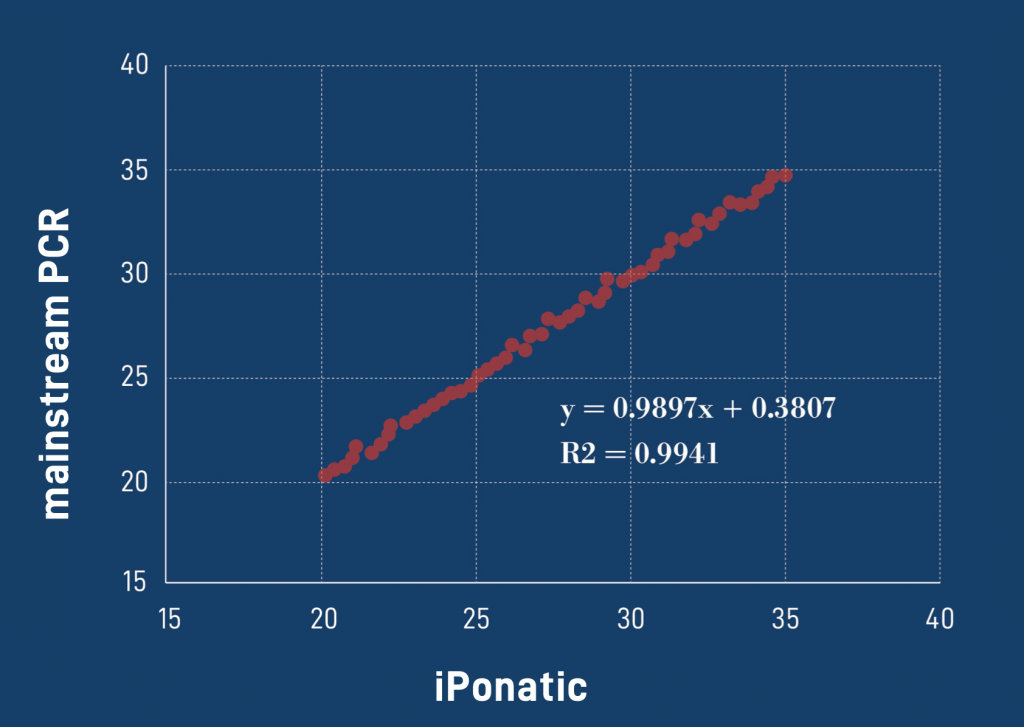
Diagnostic results consistent with international mainstream PCR instruments
Parameters
Model S-Q31A S-Q31B Detection platform Real-time PCR Detection module 1 amplification module 4 amplification modules Temperature control Liquid metal coated ceramic heating / Air cooling technology Heating rate ≥6.0℃ /s (from 50℃ to 95℃ ) Cooling rate ≥2.0℃ /s (from 95℃ to 50℃ ) Excitation light source LED Detector High sensitivity photodiode Applicable dyes FAM VIC ROX CY5 Sensitivity Can detect single copy gene Electrical specification AC 100-200V 50/60Hz Dimension 230×284×376 mm (L×W×H) 336.5 × 280 × 435 mm (L×W×H) Weight 9.7Kg 13Kg Qualification CE Test menu
Respiratory Infections : SARS-CoV-2, SARS-CoV-2/FluA/B, MP, ADV, BP.... HPV Infections : HrHPV, HPV 13+2, HPV 16/18, HPV 6/11.... Children's health : EBV, HCMV.... STIs : CT/UU/NG, CT, UU, NG, HSV-2.... Other test projects are under developmentReferences
[1]. Kozel TR, Burnham-Marusich AR. Point-of-care testing for infectious diseases: Past, present, and future. J Clin Microbiol 2017;55:2313-20.
[2]. Paige M.K. Larkin, Omai B. Garner. Molecular Point-of-Care Testing in Clinical Laboratories Laboratorians can lead a new era in rapid testing with expertise in quality control and result interpretation. Clinical Laboratory News. JUL.1.2020
-


MA-6000 Real-Time Quantitative Thermal Cycler
PCR InstrumentsBrief
The MA-6000 Real-Time Quantitative Thermal Cycler has spent many years in the research and development phase, but it was worth the wait as this advanced technology will help you treat more patients and cut down your waiting times with its ability to process up to 96 samples at one time. Our new Real-Time fluorescence quantitative PCR system MA-6000 is equipped with innovative hardware, structure and optimised software to deliver higher quality results.Features
Temperature Control Technology and Innovation MA-6000 employs a six independent temperature control module, associated with an infrared environmental scanning and monitoring system for temperature control and secured precision for the thermal block. A technologically designed thermal gradient customises twelve thermal conditions for multiple reaction actions in scientific research, maximising convenience for your research and scientific experiments. The MA-6000’s platinum sensor prevents temperature overshoot and undershoot, homogenised temperature variance which in tandem offer customers reliably excellent and repeatable data output. The World's Leading Optical Detection Technology’s Built-In Advantages A world leading technology of optical conduction and detection is applied to MA-6000. A highly functional heat-resistance optical fibre conducts full-spectrum halogen light source to samples without attenuation. The emission fluorescence signals are synchronously collected by monochrome CCD, which together with its cooling system, physically eliminate dark current on the detection array. MA-6000 ensures extraordinary detection sensitivity, extends the application of thermal cycler from nucleic acid to biotinylated protein and expands a brand-new path for multiplex diagnosis in clinical field.Specifications
PCR Module Sample capacity 96×200ul tubes / 12 x 8-well PCR strips / l x 96-well plate Temperature range 4-100 ℃ Maximum ramp rate 3.5 ℃/s for heating; 3.2℃/s for cooling Temperature accuracy ± 0. 1 ℃ Temperature uniformity ±0.25℃ Temperature monitoring method 6 independent zones for temperature surveillance Gradient capability Yes Excitation light source Full spectrum halogen lamp(5 years warranty) Excitation spectrum 380-780nm Excitation channels Built-in 6(including I extension channels) Fluorescence dyes/probes FAM / SYBR Green I / VIC / HEX / TET / Cy3 / Cy3.5 / JOE / Yellow 555 / ROX / Texas Red / Cy5 / Cy5.5 / LC Red / Tamara Detection channels 96 two-way heat-resistance optical fibers Data resolution 5.000-10.000 copies with 99.8% confidence; 1.5 times differentiation for single reaction Thermo Cycler Power supply 100-240V Frequency 50-60Hz Dimensions(WxHxD)(cm) 54.8×38.8×28.8 Weight 23kg Software Win7, Win8, Win10, etc. Qualification CE -


SLAN-96P Real-Time PCR System
PCR InstrumentsFeatures
Precise Temperature Control System: 1. The SLAN system adopts Peltier technology which can accurately and quickly control increases and decreases of temperature with a precision of ±0.1℃ and features long service life, no noise and no pollution, etc.; 2. Multiple-point temperature control technology ensures the uniformity between different wells. The temperature correction technology guarantees the accuracy of temperature and thus ensures the accuracy of assay result; 3. PCR tube temperature-control technology guarantees the accuracy of actual temperature of reagent in the tube and enables the SLAN to easily adjust for different reactions using different volumes. Sensitive Photoelectric Detection System 1. High sensitivity photoelectric detector with no background noise which is fast and stable; 2. Lifetime maintenance-free ultra-bright LED cold light source with a large signal value and high stability; 3. Unique optical fiber conduction technology that greatly improves fluorescence collection efficiency and avoids fluorescence interference between neighboring tubes; 4. The system automatically selects best gain value for each tube without the need for user configuration. The SLAN®has an extremely broad measuring range for fluorescence; 5. Extremely high detection sensitivity with an extremely low fluorescence background value. It's not necessary for users to make optical correction or background correction.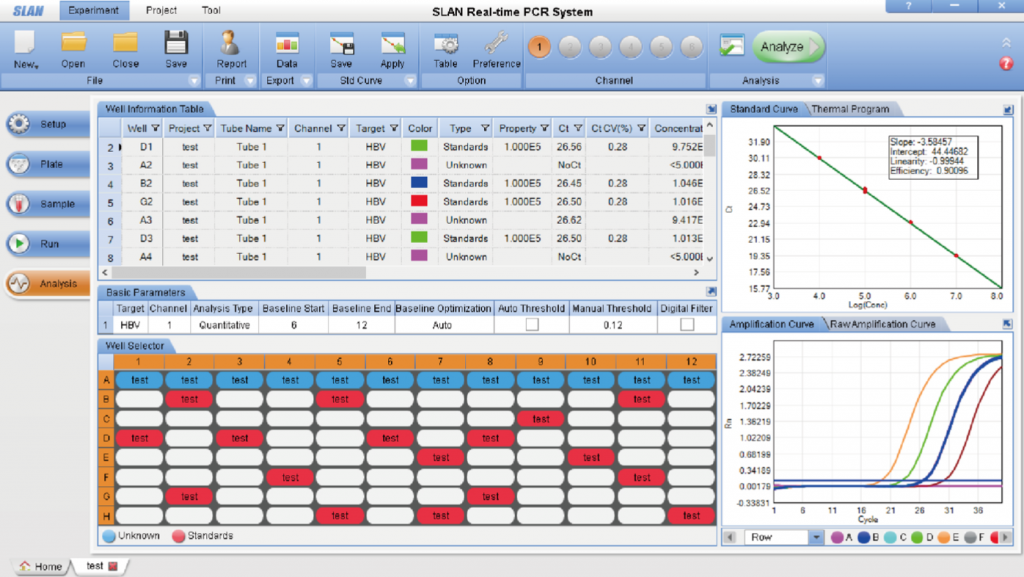
SLAN-96P Multi-tasking Software Interface (analysis screen)
Unique Electronic Automatic Hot Lid System 1. Flexible heating technology ensures uniform hot lid temperature; 2. The electronic automatic hot lid has an adjustment function for different 0.2ml reaction tubes to ensure even tube pressure; 3. Once the assay starts, the hot lid has automatic locking function to prevent assay failure following accidental opening. Other functions· Power-off protection · Automatic saving result · Export of result · Real-time data display · Quantitative and qualitative analysis · File encryption · Automatic baseline optimization · Digital filtering · Search function · Report sheet print and edit · Melt curve function Specifications
Product model SLAN-96P Sample capacity 96 wells (dual 48/48 reaction module) Sample size 15-50mL Consumable 0.2mL PCR tubes, 8-tube strips, 48-well plates Parallel running mode Dual reaction blocks/running 2 tests independently Temperature range 4-99°C Max ramp rate 4.0°C/sec Temperature accuracy ±0.1°C Temperature uniformity ±0.1°C Thermal cycling system Peltier-based system Temperature control mode Tube control/block control Light Source LED (maintenance-free) Detector High sensitivity photoelectric sensor Sensitivity 1 copy Linearity range 100-1010 Resolution Can discriminate between 1000 copies and 2000 copies Excitation CH1 470nm CH2 530nm CH3 580nm CH4 630nm CH5/CH6 custom-made Emission CH1 510nm CH2 565nm CH3 620nm CH4 665nm CH5/CH6 custom-made Dyes and probes CH1 FAMTM /SYBRGreen® CH2 VIC®/HEX/J0ETM /TET CH3 ROX/TexasRed® CH4 CY5TM Hot-lid 30°C~108°C (default105°C, adjustable) electronic automatic hot-lid Power supply 230VAC, 50Hz Power consumption 850VA Dimensions 380mm x 520mm x 250mm (WxDxH) Weight 18Kg Communication RS232, USB Qualification CE -


Natch 48 Nucleic Acid Extraction System
Extraction InstrumentsExtremely simple workflow

Specifications
Instrument model Natch 24/48/96 Sample type Serum, plasma, whole blood, secretions, exfoliated cells, tisues, throat swabs, anal swabs, urine, feces, etc. Extraction technology Advanced magnetic beads technology Sample type Volume 1-96 tests/batch Extraction Time 10-60 minutes each batch Working volume 30-2000uL Recovery rate of magnetic beads >95% Blending mode Vortex omnidirectional liquid mixing Temperature control range Adjustable from roomtemperature to 125℃ Operation 7-inch touch screen Storage Up to 120 groups of programs can be used. Size and weight 655x655x520 mm (L x W x H), weight: 65kg Qualification CE Extraction kit components
No. Spec. Product Name S10016E 48T/kit Nucleic Acid Extraction-Purification Kit (Magnetic beads method) Available projects
Sexually Transmitted Diseases : CT, NG, UU, HSV-2, HSV I/2 Women's Health : hrHPV and hrHPV genotyping Respiratory Tract Disease : 6RP, TB, MP, RSV, ADV, BP Gastrointestinal Diseases : HFMD Prenatal and Postnatal care : CMV, EBV Public Health : SARS-CoV-2, Influenza A/B, HINI, H7N9 Customerized Projects -


Natch CS2 Fully Automated Nucleic Acid Extraction System
Extraction InstrumentsExtremely simple workflow

Features
1. Efficient anti-contamination
2. Super magnetic, effective adsorption
- Well-organized countertop layout
- Sample area is separated from clean area
- Effective UV decontamination
- Anti-contanmination function of moving path
3. Excellent sample addition function
- Super 3-D magnetic adhesion technology
- Permanent magnetic mode
- 117 magnetic racks, 4 magnetic bars in each well
4. Easy-to-use software
- Automatic liquid level detection
- Clot detection
- Tip detection
- Air-prof detection
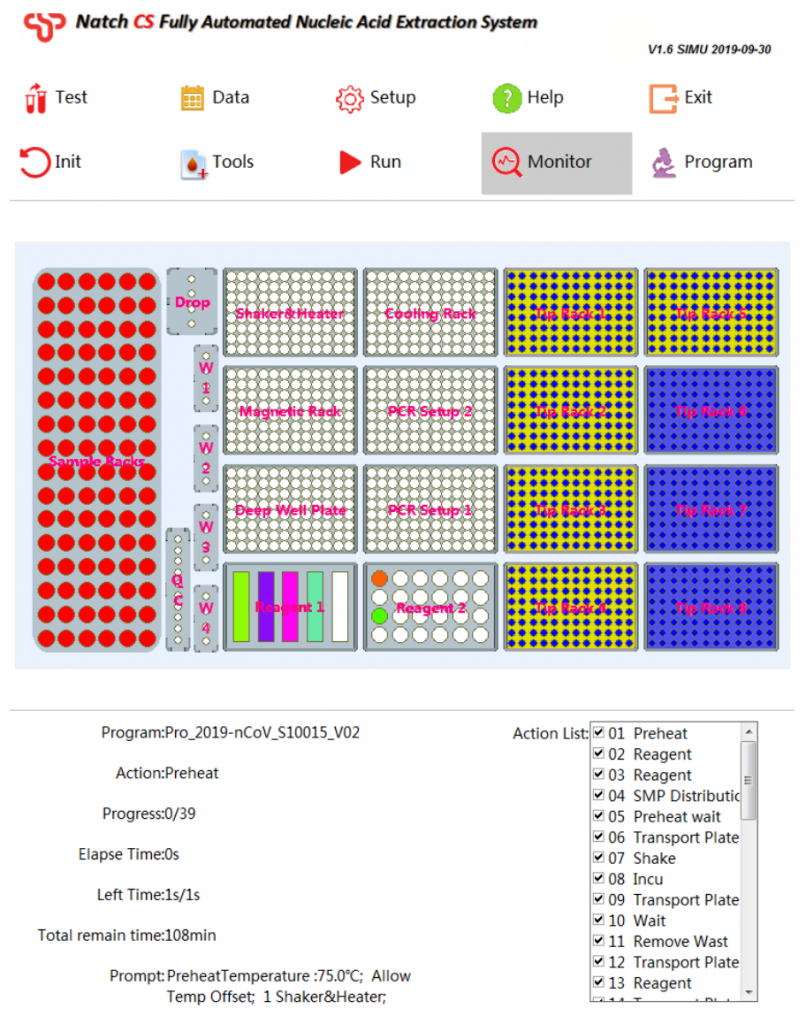
- Preset programs, one-key selection
- Prompt the placement of reagents
- Display the status of reagents and consumables
- Real-time display of remaining time
- Show real-time experimental procedures
- Permits LIS compatibility
Specifications
Instrument model S-S13A Sample type Serum, plasma, whole blood, secretions, exfoliated cells, tissues, nasopharyngeal swab, oropharyngeal swab, urine, feces, etc. Sample tube specifications Compatible with various original sample tubes Extraction Principle one-tube fast release technology (OT) Advanced magnetic beads technology (MB) Sample type Volume OT: 1-96 tests/batch; MB: 1-96 tests/batch Extraction Time OT of 96 tests ≤ 30 minutes; MB of 96 tests ≤ 90 minutes Pipetting range 10~1000 μL Level detection Pressure detection / capacitance detection, automatic detection of liquid level Clot detection Pressure detection, automatic detection of liquid aspiration and liquid clogging Sample loading function Automatic liquid level detection Clot detection Tips detection Air-prof detection Magnetic field control Permanent magnet mode Patented design Super 3-D magnetic adhesion technology Blending mode Vortex omnidirectional liquid mixing Temperature control range Adjustable from room temperature to 100℃ UV disinfection Timed opening and closing Barcode Scanning Sample scanning system Supported multiple barcodes Language Simplified Chinese / English Display Method 10.4 inches touch screen Communication Interface USB, RS232, network ports, HDMI Dimensions and Weight 1320×850×1600mm (LxWxH), weight: 350Kg Power supply Input: AC 100-240V 50/60Hz 350 VA Qualification CE Extraction kit components
No. Spec. Product Name S1013E 48T/kit Sample Release Reagent S1014E 24T/Kit, 48T/kit Sample Release Reagent S1002E 24T/kit Nucleic Acid (DNA/RNA) Extraction or Purification Kit (Magnetic beads method) S1006E 48T/kit Multi-type Sample DNA/RNA Extraction-Purification Kit (Magnetic beads method) S10016E 48T/kit Nucleic Acid Extraction-Purification Kit (Magnetic beads method) Available projects
Hepatitis and AIDS : HBV, HCV, HIV Sexually Transmitted Diseases : CT, NG, UU, HSV-2, HSV I/2 Women's Health : hrHPV and hrHPV genotyping Respiratory Tract Disease : 6RP, TB, MP, RSV, ADV, BP Gastrointestinal Diseases : HFMD Prenatal and Postnatal care : CMV, EBV Public Health : SARS-CoV-2, Influenza A/B, HINI, H7N9 Customerized Projects -


S1006E Multi-type Sample DNA/RNA Extraction-Purification Kit
DNA/RNA Extraction ReagentsBrief
Multi-type Sample DNA/RNA Extraction-Purification Kit (Suprall extraction reagent) is an innovative product based on Sansure advanced magnetic beads technology platform , which can accomplish the unified extraction of nucleic acids in various gene detection applications and samples to provide comprehensive solutions for nucleic acid extraction , can be applied in infectious disease diagnosis, genetic disease screening, birth defect prevention, tumor screening, individualized diagnosis and treatment, detection of disease susceptibility genes, sequencing services and other fields.Parameters
Qualification CE Kit components
No. Product Name Spec. S1006E Multi-type Sample DNA/RNA Extraction-Purification Kit 24T/kit -

Nucleic Acid (DNA/RNA) Extraction or Purification Kit
DNA/RNA Extraction ReagentsBrief
Nucleic Acid (DNA/RNA) Extraction or Purification Kit developed based on Sansure advanced magnetic beads technology platform,using exclusive modified super-paramagnetic nano-beads to absorb sample DNA/RNA , only needs a simple step of washing to obtain high purity nucleic acid, combined with the "DNA/RNA elution-free " technology and integrated high-efficient amplification system, to achieve DNA/RNA amplification detection with magnetic beads.Parameters
Qualification CE Kit components
No. Product Name Spec. S1002E Nucleic Acid (DNA/RNA) Extraction or Purification Kit 24T/kit S10011E Nucleic acid (DNA/RNA) extraction or purification kit 24T/Kit, 48T/kit S10016E Nucleic Acid Extraction-Purification Kit 48T/kit, 96T/kit -


Sample release reagent
DNA/RNA Extraction ReagentsBrief
Sample release reagent developed based on the Sansure one-tube fast release technology platform. Adopting Sansure patent nucleic acid release technology, can quickly lyse pathogens at room temperature, no need heating, centrifuging or replacing tubes, the sample DNA/RNA can be extracted quickly through simple operations. Sample Release Reagent is used for the pretreatment of nucleic acids, to release the nucleic acids from specimens, then the released nucleic acids can be used for clinical in vitro diagnosis or used for the detection through equipment.Parameters
Qualification CE Kit components
No. Product Name Spec. S1011E Sample Release Reagent 48T/kit S1013E Sample Release Reagent 48T/kit S1014E Sample Release Reagent 24T/Kit, 48T/kit -


S3053E EV/EV71/CA16 – Enterovirus/Coxsackievirus A16/Enterovirus 71 RNA Diagnostic Kit
Gastrointestinal Infections (GIIs)Brief
Hand-foot-and-mouth disease is a common childhood disease caused by enteroviruses. Most of the patients will have a fever and get vesicle rash in the mouth or on the hand and foot. A few patients will get cephalomeningitis, cerebritis, neurogenic pulmonary edema and myocarditis, etc. Some patients may get sequelae of this disease or even death. The viruses that can cause hand-foot-and-mouth disease include Coxsackievirus, new-type enterovirus and ECHO virus, of which the most common types are Coxsackievirus A16 and Enterovirus 71. The laboratory diagnosis methods mainly include virus isolation, nucleic acid detection, etc. The Enterovirus/Coxsackievirus A16/Enterovirus 71 RNA Diagnostic Kit (PCR-Fluorescence Probing) is intended to detect Enterovirus, Coxsackievirus A16 and Enterovirus 71 in throat swab by applying real-time quantitative PCR technique. The detection results can be used to distinguish Coxsackievirus A16 and Enterovirus 71.Parameters
Product features Parameter Specimen Type throat swab Technical Platform Advanced magnetic beads technology Target pathogen EV, EV71, CA16 Internal Control Lentivirus PCR Instrument ABI7500,SLAN-96P Amplification Time 100 min Sensitivity 1000 copies/mL Spec. 24T Qualification CE -


S3014E HCMV – Human Cytomegalovirus DNA Quantitative Fluorescence Diagnostic Kit
Other InfectionsBrief
Human cytomegalovirus (HCMV), also called cell inclusion body virus, is a double helix DNA virus and belongs to β genus of herpes virus family, which causes infected cells to enlarge and has a huge intranuclear inclusion. The ways of infection of HCMV is mainly through contact, blood transfusion, intrauterine and birth canal, and the infections are commonly found in fetus, newborns, pregnant women, etc. If the pregnant women are infected, it may cause their newborns congenital monstrosity. When the organism immune deficiency or immune system is under inhibitory state, people can be easily infected by HCMV, such as the patients receiving immunosuppressive therapy after transplantation of organ, the patients receiving malignant tumor chemotherapy and AIDS patients, etc. If these patients are infected by HCMV, it usually causes high mortality and serious diseases. Clinical tests suggest that HCMV infection hasn’t specific manifestations and it causes harm to multiple organs, especially the liver and lung. A statistical analysis of positive and negative rate of HCMV in various kinds of clinical indications shows HCMV infection is probably related with various diseases, such as Cytomegalovirus hepatitis, Infant hepatitis syndrome, liver dysfunction, pneumonia, Bronchitis, Upper respiratory tract infections, enteritis, enterocolitis, diarrhea, hematemesis, heart failure, etc. The Human Cytomegalovirus DNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of HCMV DNA in human urine, serum and peripheral blood samples. It is intended for use as an aid in the diagnosis of an HCMV infection and for observing drug efficacy.Parameters
Product features Parameter Specimen Type urine, serum, and peripheral blood. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen HCMV Internal Control cloning plasmid PCR Instrument ABI7500, Roche LC 480, Stratagene Mx3000P, SLAN-96P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T Qualification CE -


S3015E EBV – Epstein-Barr virus DNA Quantitative Fluorescence Diagnostic Kit
Other InfectionsBrief
Epstein-barr virus (EBV) is known to be the first virus that is definitely related with human tumors. EBV infection mainly causes infectious mononucleosis in children and tumor-related diseases such as Burkitt’s lymphoma, lymphoid tissue hyperplasia in immunocompromised individuals, primary lymphoma, empyema associated lymphoma, T-cell lymphoma, NK cell lymphoma/leukemia, Hodgkin’s disease, nasopharyngeal carcinoma, stomach cancer, lymphoid epithelial tissue cancer, smooth muscle tumors, etc. The Epstein-Barr virus DNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the quantification of Epstein-Barr Virus (EBV) DNA in peripheral blood. Test result can be used as an aid in the diagnosis of an EBV infection and in observation of drug efficacy.Parameters
Product features Parameter Specimen Type peripheral blood. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen EBV Internal Control cloning plasmid PCR Instrument ABI7500, Roche LC 480 and Stratagene Mx3000P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T Qualification CE -

C003E BCI – Nucleic Acid Test Kit for HBV, HCV, HIV (Type1+2)
Blood-borne Infections (BBIs)Brief
Nucleic Acid Test Kit for HBV, HCV, HIV (Type1+2), is based on real-time fluorescence PCR technology and used for nucleic acid qualitative detection of HBV, HCV, HIV1+2 in plasma. This kit is intended for use as a blood donor screening test to detect HBV DNA, HCV RNA, HIV-1 RNA and HIV-2 RNA in pooled or individual sample from healthy blood donors, blood donors of various components (red blood cells, platelets and plasma) and other types of blood donors. All plasma to be tested can be screened as individual samples or tested in pools after mixing with each equal aliquots according to routine serological screening results HBV, HCV and HIV samples. For the pooled sample showed positive test results, carry out individual testing, the individual test result should be used as the final test result of this sample. The test results of this diagnostic kit can distinguish reactivity of between HBV, HCV and HIV.Advantages
High sensitivity- HBV 3IU/mL
- HCV 10IU/mL
- HIV 45IU/mL
- 576 tests/ 5h(pooling)
- 45 tests / 4.5h(single
- HBV A-H
- HCV 1-6
- HIV-1(M/N/O)and HIV-2
- Four tests for a tube of samples
- Direct discriminating positive
- Extraction and amplification process
- No need to be on duty
- One extractor + one amplifier
- Less consumable material consumption
Features
Sample preparation and extraction in one module Fully automatic sample preparation and nucleic acid extraction in one module to build up a integrated reaction system Flexible testing mode 6 pooling sample testing or individual donor testing (IDT) supported Advanced magnetic beads technology Nanometer-level beads enable beads-in-PCR amplification ensuring maximum nucleic acid template Patented magnetic beads lateral suction technology Thorough waste liquid removal allows minimal magnetic beads loss Sophisticated sample information processing Automatic recognition of the sample barcode & sample tracking and archiving table generation available High throughput 558/45 samples result output in one time Minimal system maintenance time Less than 20 minutes startup with fewer maintenance tasks Excellent accuracy A total number of 105,124 blood bags which confirmed as negative by serological testing were tested against some referential screening kits. 15 false-negative results in reference tests were found.Parameters
Qualification CE -

S3034E HCV Genotype – Hepatitis C Virus Genotype Diagnostic Kit
Blood-borne Infections (BBIs)Brief
By applying real-time PCR technology, the Hepatitis C Virus Genotype Diagnostic Kit (PCR-Fluorescence Probing) is designed for qualitative identification of HCV genotypes (including genotypes 1b, 1, 2, 3, 4, 5 and 6) from HCV RNA positive samples. The test results can be used as an aid in the identification of HCV genotypes and determination of an appropriate therapeutic treatment indicated for the above listed gentoypes. The results can be used only for clinical reference, and cannot be used as the only evidence for adjusting therapeutic drugs. Clinical symptoms and other laboratory test results should also be considered to comprehensively determine the patients treatment.Parameters
Product features Parameter Specimen Type serum Extraction Platform Magnetic beads technologies Genotype 1b, 1(1b, 1a), 2 (2a), 3 (3a, 3b), 4 (4a), 5 (5a), 6 (6a) Internal Control Plasmid PCR Instrument Mx3000P,Slan 96P Sensitivity 1000 IU/mL Spec. 12T Qualification CE -


S3119E HCV Ultra – Hepatitis C Virus RNA Quantitative Fluorescence Diagnostic Kit
Blood-borne Infections (BBIs)Brief
Hepatitis C Virus RNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the quantification of HCV RNA in human serum or plasma. It is intended for use as an aid in the diagnosis of an HCV infection and observing drug efficacy. Hepatitis C is mainly caused by HCV infection and transmitted through blood. Chronic infection of HCV can lead to chronic inflammation of liver and fibrosis, and some patients may develop into liver cirrhosis, even Hepatocellular Carcinoma (HCC). It has huge harms on patients’ health and life quality, and has become a severe social and public health issue.Parameters
Product features Parameter Specimen Type Serum or plasma Extraction Platform Advanced magnetic beads technology Genotype HCV 1-6 genotype Internal Control Lentivirus particles PCR Instrument SLAN-96P, ABI7500, Roche cobas 480 Sensitivity 12 IU/mL Linear range 25—1.0E+08 IU/mL Spec. 24T -

S3013E HBV fast – Hepatitis B Virus DNA Quantitative Fluorescence Diagnostic Kit
Blood-borne Infections (BBIs)Brief
Hepatitis B Viral (HBV) DNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the quantification of HBV DNA in human serum or plasma. It can be used to evaluate antiviral treatment and monitor the therapeutic effect by monitoring HBV DNA baseline levels and changes in patient blood. Test results should not be taken as the only indicator for evaluation of diseases, but to be combined with patients clinical symptoms and other laboratory tests to analyze the diseases.Parameters
Product features Parameter Specimen Type Serum or plasma Extraction Platform One-tube fast test release Genotype HBV genotype A-H Internal Control Plasmid Compatible Instrument ABI7300 , Stratagene Mx3000P , Roche Light Cycler 480 , ABI7500, SLAN-96P and QuantStudio 5 Sensitivity 30 IU/mL Linear range 100—5.0E+09 IU/mL Spec. 24T, 48T -

S3118E HBV Ultra – Hepatitis B Virus DNA Quantitative Fluorescence Diagnostic Kit
Blood-borne Infections (BBIs)Brief
Hepatitis B Virus DNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) (HBV Ultra) is an in vitro nucleic acid amplification test for the quantification of Human HBV DNA in human serum or plasma. It is intended for use as an aid in diagnosing an HBV infection and observing drug efficacy.Performance
Advanced magnetic beads technology HBV DNA detection and viral load measurement are essential for treatment decisions and patient monitoring. Sansure's HBV Ultra uses the advanced magnetic beads technology to extract HBV DNA from clinical Plasma. Our technology achieved high sensitivity and wide linear range detection to meet of clinical diagnosis and follow-up needs. Highly conservative primer probe design Sansure primers and probes target select the highly conservative S gene of HBV, which can cover A-H genotypes and avoid missed detection. High-efficient quality control system HBV Ultra uses UNG enzyme + dUTP system to remove carry-over contamination to avoid a false positive result. HBV Ultra uses Internal Control HBV Ultra uses Internal Control is the full name to whole-process the HBV extraction and amplification process to avoid false negative results.Parameters
Product features Parameter Specimen Type Serum or plasma Extraction Platform Advanced magnetic beads technology Genotype HBV genotype A-H Internal Control Pseudoviruses PCR Instrument SLAN-96P, ABI7500, Roche cobas 480 Sensitivity 5 IU/mL Linear range 20—2.0E+09 IU/mL Spec. 48T -

S3017E HPV 6,11 – Human papillomavirus (Type 6 and 11) DNA Fluorescence Diagnostic Kit
HPV InfectionsBrief
HPV (Type 6 and 11) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of low-risk HPV (type 6/11) DNA in reproductive tract secretions. It is intended for use as an aid in the diagnosis of an HPV (type 6/11) infection and patients with clinically suspected genital warts.Parameters
Product features Parameter Specimen Type reproductive tract secretions Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 6, 11 Internal Control β--globin gene PCR Instrument ABI7500,QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II,iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -

S3019E HPV 16, 18 – Human papillomavirus (Type 16 and 18) DNA Fluorescence Diagnostic Kit
HPV InfectionsBrief
HPV (Type 16 and 18) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (Type 16 and 18) DNA in reproductive tract secretions. It is intended for use as an aid in the diagnosis of an HPV (Type 16 and 18) infection, and the early screening of cervical cancer.Parameters
Product features Parameter Specimen Type reproductive tract secretions Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 16, 18 Internal Control β--globin gene PCR Instrument ABI7500,QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II,iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -

S3360E HPV 15HR – 15 High-risk Human Papillomavirus Nucleic Acid Diagnostic Kit
HPV InfectionsBrief
15 High-risk HPV DNA Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68) present in exfoliated cells from females’ cervix. The test results can be used as an aid in the diagnosis of a high-risk HPV infection.Parameters
Product features Parameter Specimen Type exfoliated cells from females’ cervix Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68 Internal Control β--globin gene PCR Instrument ABI7500, QuantStudio 5, MA-6000, SLAN-96P, LightCycler 480, Stratagene Mx3000P, iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 1000 copies/mL Spec. 48T, 12-P Qualification CE -


S3027E HPV G15 – High-risk Human Papillomavirus DNA (Genotype) Diagnostic Kit
HPV InfectionsBrief
This High-risk HPV DNA (Genotype) Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68) present in exfoliated cells from females’ cervix. The test results can be used for identification of HPV genotypes.Parameters
Product features Parameter Specimen Type Exfoliated cells from females’ cervix Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68 Internal Control β--globin gene PCR Instrument SLAN-96P, ABI 7500, Roche LC 480 and QuantStudioTM 5 , Stratagene Mx3000P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 24T/48T Qualification CE -


S3057E HPV 13+2 – Human Papillomavirus DNA Diagnostic Kit
HPV InfectionsBrief
Base on the study results by WHO International Agency for Research on Cancer (IARC) and other international organizations, the 13 kinds of genotypes including HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 are classified as high-risk HPV, and the 5 kinds of genotypes including HPV26, 53, 66, 73, 82 are classified as medium-risk HPV. Human Papillomavirus DNA Diagnostic Kit (PCR-Fluorescence Probing) chooses the above 13 kinds of high-risk genotypes and two popular kinds of medium-risk genotypes that are HPV 53 and 66 to be the target genotypes, in order to guarantee that the kit is capable of cervical carcinoma screening and risk evaluation. Moreover, it is clearly indicated that the females at the age of 30 or above who have no abnormal cervical cytology but the HPV detection is positive, especially for HPV16 and HPV18 infected females, should get vaginoscopy immediately. Therefore, the diagnostic kit can be used for detection of the 15 kinds of target genotypes and also subtype identification of HPV16 and HPV18.Parameters
Product features Parameter Specimen Type Exfoliated cells from females’ cervix Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types Type 16+18+31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68 Internal Control β--globin gene PCR Instrument ABI7500, MA-6000, SLAN-96P, QuantGene 9600 , iPonatic S-Q31A&B, S-Q36A Amplification Time 70 min Sensitivity 200 copies/mL Spec. 48T, 24-P Qualification CE -

S3002E HSV-2 – Herpes Simplex Virus Type 2 DNA Fluorescence Diagnostic Kit
Reproductive Tract InfectionsBrief
Herpes Simplex Virus Type 2 (HSV-2) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is used to detect the presence of HSV-2- DNA in samples such as male urethral swab and female cervical swab. The detection result can be used as an aid in the diagnosis of an HSV-2 infection. The test provides a molecular diagnostics-based solution for early diagnosis of venereal disease and for preliminary screening of high-risk venereal disease groups.Parameters
Product features Parameter Specimen Type Genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen HSV-2 Internal Control Recombinant plasmid PCR Instrument ABI7500,QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II,iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -


S3004E UU – Ureaplasma Urealyticum DNA Fluorescence Diagnostic Kit
Reproductive Tract InfectionsBrief
The Ureaplasma Urealyticum (UU) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is used to detect the presence of UU-DNA in samples such as male urethra swab and female cervical swab. The detection result can be used as an aid in the diagnosis of a UU infection. The test provides a molecular diagnostics-based solution for early diagnosis of venereal disease and for preliminary screening of high-risk venereal disease groups.Parameters
Product features Parameter Specimen Type Genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen UU Internal Control Recombinant plasmid PCR Instrument ABI7500,QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II,iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -


S3003E NG – Neisseria Gonorrhoeae DNA Fluorescence Diagnostic Kit
Reproductive Tract InfectionsBrief
Neisseria Gonorrhoeae (NG) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is used to detect the presence of NG-DNA in samples such as male urethral swab and female cervical swab. The detection result can be used as an aid in the diagnosis of an NG infection. The test provides a molecular diagnostics-based solution for early diagnosis of venereal disease and for preliminary screening of high-risk venereal disease groups.Parameters
Product features Parameter Specimen Type Genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen NG Internal Control Recombinant plasmid PCR Instrument ABI7500, QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II, iPonatic S-Q31A&B, S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -

S3001E CT – Chlamydia Trachomatis DNA Fluorescence Diagnostic Kit
Reproductive Tract InfectionsBrief
Chlamydia Trachomatis (CT) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) applies to detect CT DNA in specimens like reproductive tract secretion. The detection result can be used for aiding diagnosis of CT infection, which provides molecular diagnosis base for early diagnosis of venereal disease and for preliminary screening of venereal disease high-risk groups.Parameters
Product features Parameter Specimen Type genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen CT Internal Control Recombinant plasmid PCR Instrument ABI 7500, Roche LC480,Stratagene Mx3000P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T Qualification CE -


S3050E CT/UU/NG – Chlamydia Trachomatis/Ureaplasma Urealyticum/Neisseria Gonorrhoeae DNA Diagnostic Kit
Reproductive Tract InfectionsBrief
Chlamydia Trachomatis/Ureaplasma Urealyticum/Neisseria Gonorrhoeae DNA Diagnostic Kit (PCR-Fluorescence Probing) is a qualitative in vitro test for simultaneous detection of Chlamydia Trachomatis, Ureaplasma Urealyticum and Neisseria Gonorrhoeae DNA in sterile calcium alginate swab specimens from male urinary tract secretion and female genital tract secretion by applying real-time fluorescence quantitative PCR technique, which can provide molecular diagnosis evidence for the early diagnosis of related sexually transmitted diseases and initial screening of high-risk STD population.Parameters
Product features Parameters Specimen Type genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen CT, UU, NG-DNA Internal Control β--globin gene PCR Instruments ABI7500,SLAN-96P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T Qualification CE -


S3113E SC2/FluA/B – SARS-CoV-2 and Influenza A/B Virus Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Most people infected with the COVID-19 virus will experience mild to moderate respiratory illness and recover without requiring special treatment. Elder people, and those with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop serious illness. Sansure COVID-19 diagnostic solutions are used to directly detect the presence of viral RNA, which will be detectable in patients before antibodies form or symptoms of the disease are present, which means the test results can tell whether or not that someone gets virus very early on in their illness. This kit can also joint-detect RNA of influenza A virus and influenza B virus.Parameters
Product features Parameters Specimen Type Oropharyngeal swab, sputum Extraction Platform One-tube fast release technology Advanced magnetic beads technology Target Genes SARS-CoV-2:ORF 1ab, N gene; influenza A:M gene; influenza B:NP gene Internal Control Rnase P gene PCR Instrument ABI7500, SLAN-96P, MA-6000, QuantGene 9600, iPonatic S-Q31A&B,S-Q36A Sensitivity 200 copies/mL Spec. 24T, 48T, 24-P Qualification CE -

S3148E SC2/Flu/RSV – SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus Multiple Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
The SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus Multiple Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) is a real-time RT-PCR test intended for the qualitative Diagnostic of nucleic acid from SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus Multiple in the nasopharyngeal swabs and oropharyngeal swabs from individuals.
As the seventh coronavirus that infects humans, the SARS-CoV-2 can cause fever, fatigue, dry cough, dyspnea and other symptoms. In severe cases, it can cause acute respiratory distress syndrome, septic shock, and even death. At the same time, the SARS-CoV-2 has a strong spreading ability and has a wide range. Influenza virus (Influenza virus) can cause acute respiratory infections, with clinical manifestations of fever, headache, myalgia, fatigue, rhinitis, sore throat and cough. Influenza viruses can aggravate underlying diseases (such as heart and lung diseases) or cause secondary bacterial pneumonia or primary influenza viral pneumonia. The elderly and people with various chronic diseases or physical weakness are prone to severe complications and mortality higher after infecting influenza. Respiratory syncytial virus (RSV) belongs to the Pneumovirus genus of the Paramyxoviridae family. It mainly causes lower respiratory tract infections such as bronchiolitis and pneumonia in infants under 6 months, as well as rhinitis , Cold and other upper respiratory tract infections in older children and adults.
Parameters
Product features Parameter Covering pathogensSARS-CoV-2, Influenza Virus and Respiratory Syncytial VirusSpecimen TypesNasopharyngeal swab and oropharyngeal swabTechnical PlatformOne-tube fast release technologyAdvanced magnetic beads technologyInternal ControlRNase P geneCompatible InstrumentABI 7500; MA-6000; SLAN®-96P; iPonatic S-Q31A/S-Q31B/S-Q36ASensitivity500 copies/mL.QualificationCE -


S3102E SC2 – Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
COVID-19 is an infectious disease caused by a newly discovered coronavirus named SARS-CoV2. Most people infected with the COVID-19 virus will experience mild to moderate respiratory illness and recover without requiring special treatment. Elder people, and those with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop serious illness. Sansure COVID-19 diagnostic solutions are used to directly detect the presence of viral RNA, which will be detectable in patients before antibodies form or symptoms of the disease are present, which means the test results can tell whether or not that someone gets virus very early on in their illness.Performance
- One-tube/fast release technology
- Up to 96 samples at one time
- Simple operation process, less specialist training
- Room temperature lysis, less contamination
- Sampling types : nasopharyngeal swab, oropharyngeal swab, alveolar lavage fluid, sputum, serum, whole blood ,Feces
- Enhance large-scale screening efficiency
- Internal control: human housekeeping gene RNase P
Parameters
Product features Parameters Specimen Type Nasopharyngeal swab, oropharyngeal swab, alveolar lavage fluid, sputum, serum, whole blood, feces Extraction Platform One-tube fast release technology Advanced magnetic beads technology Internal Control Rnase P gene PCR Instrument ABI7500, QuantStudioTM 5, SLAN-96P, MA-6000, Bio-Rad CFX-96, QuantGene 9600, LightCycler 480, iPonatic S-Q31A&B,S-Q36A Sensitivity 200 copies/mL Spec. 24T, 48T, 24-P Qualification CE -

S3121E SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay)
Respiratory Tract InfectionsBrief
SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay) is intended for the qualitative detection of the SARS-CoV-2/InFluA/InFluB nucleocapsid protein in human nasopharyngeal or oropharyngeal swabs. Test results will be available for reading in 15-20 minutes. Positive results indicate the presence of viral antigens. This kit is for in vitro diagnostic use.
Features
- Accurate LOD 80TCID50 /ml, Specificity 100%
- Convenient No instrument needed, easy to operate
- Fast Result available in only 15-20 min
Instruction
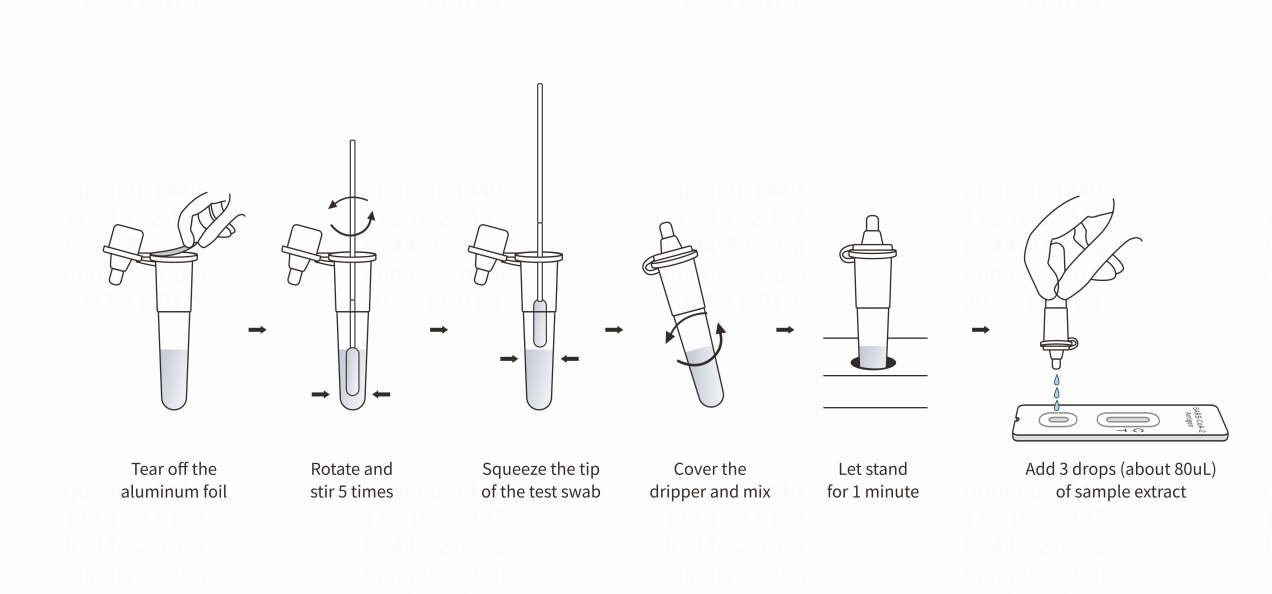
Result interpretation
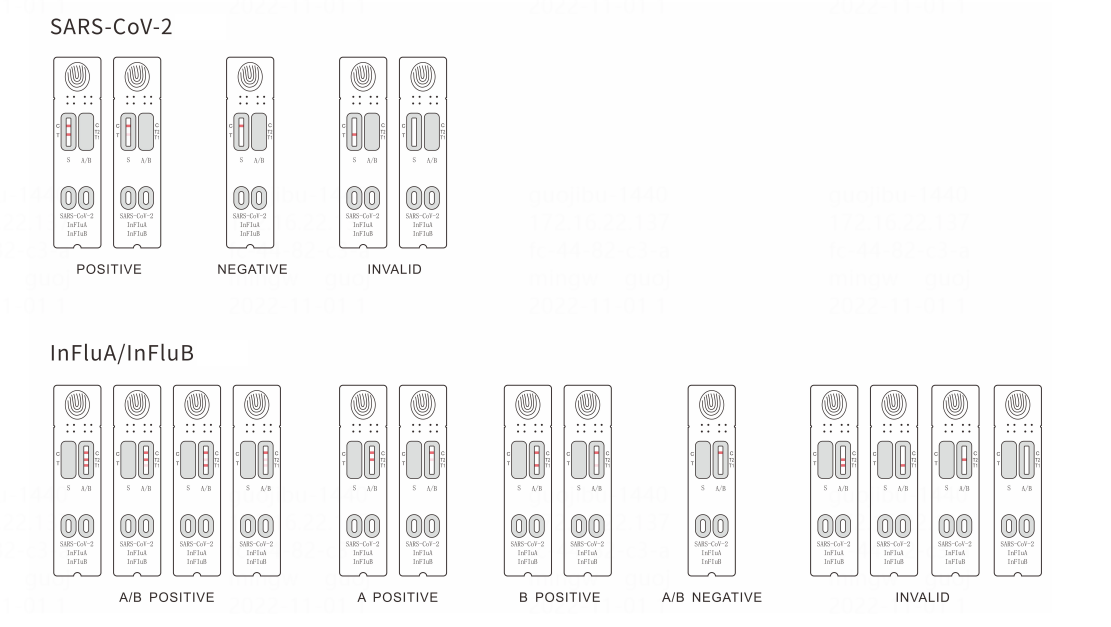
Parameters
Qualification CE Kit components
No. Product Name Spec. S3121E-25SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay)25 Test The kit components:
- SARS-CoV-2/InFluA/InFluB Antigen Test Cassette (individually in a foil pouch with desiccant)
- Sample Extraction Buffer
- Swab
