-

C004E BCI – Nucleic Acid Test Kit for HBV, HCV, HIV (Type1+2)
Blood-borne Infections (BBIs)Brief
Nucleic Acid Test Kit for HBV,HCV,HIV(Type1+2), is based on real-time fluorescence PCR technology and used for nucleic acid qualitative detection of HBV, HCV, HIV1+2 in plasma. This kit is intended for use as a blood donor screening test to detect HBV DNA, HCV RNA, HIV-1 RNA and HIV-2 RNA in pooled or individual sample from healthy blood donors, blood donors of various components (red blood cells, platelets and plasma) and other types of blood donors. All plasma to be tested can be screened as individual samples or tested in pools after mixing with each equal aliquots according to routine serological screening results HBV, HCV and HIV samples. For the pooled sample showed positive test results, carry out individual testing, the individual test result should be used as the final test result of this sample. The test results of this diagnostic kit can distinguish reactivity of between HBV, HCV and HIV.Advantages
High sensitivity- HBV 2.41 IU/mL
- HCV 12.38 IU/mL
- HIV-1 31.68 IU/mL
- HIV-2 48.34 IU/mL
- 576 tests/ 5h(pooling)
- 96 tests / 4.5h(single)
- HBV genotypes A-H
- HCV genotypes 1-6
- HIV-1 group M/N/O
- HIV-2
- Four tests for a tube of samples
- Direct discriminating positive
- Extraction and amplification process
- No need to be on duty
- One extractor + one amplifier
- Less consumable material consumption
Features
Sample preparation and extraction in one module Fully automatic sample preparation and nucleic acid extraction in one module to build up a integrated reaction system Flexible testing mode 6 pooling sample testing or individual donor testing (IDT) supported Advanced magnetic beads technology Nanometer-level beads enable beads-in-PCR amplification ensuring maximum nucleic acid template Patented magnetic beads lateral suction technology Thorough waste liquid removal allows minimal magnetic beads loss Sophisticated sample information processing Automatic recognition of the sample barcode & sample tracking and archiving table generation available High throughput 558/96 samples result output in one time Minimal system maintenance time Less than 20 minutes startup with fewer maintenance tasks Excellent accuracy A total number of 105,124 blood bags which confirmed as negative by serological testing were tested against some referential screening kits. 15 false-negative results in reference tests were found.Parameters
Qualification CE -
S3120E-H-SARS-CoV-2 Rapid Antigen Test for Self-testing
Respiratory Tract InfectionsBrief
The SARS-CoV-2 Rapid Antigen Test (Immunochromatography Assay) for self-testing is authorized for home use with self-collected nasal swab samples to directly detect antigen of SARS-CoV-2 virus. With the help of this kit, people without professional training can also easily acquire their COVID-19 test result within 15-20 minutes.Features
- Accurate Sensitivity 94.55%, Specificity 100%
- Convenient No instrument needed, easy to operate
- Fast Result available in only 15-20 min
- Flexible Get tested anytime when you are free
Instruction
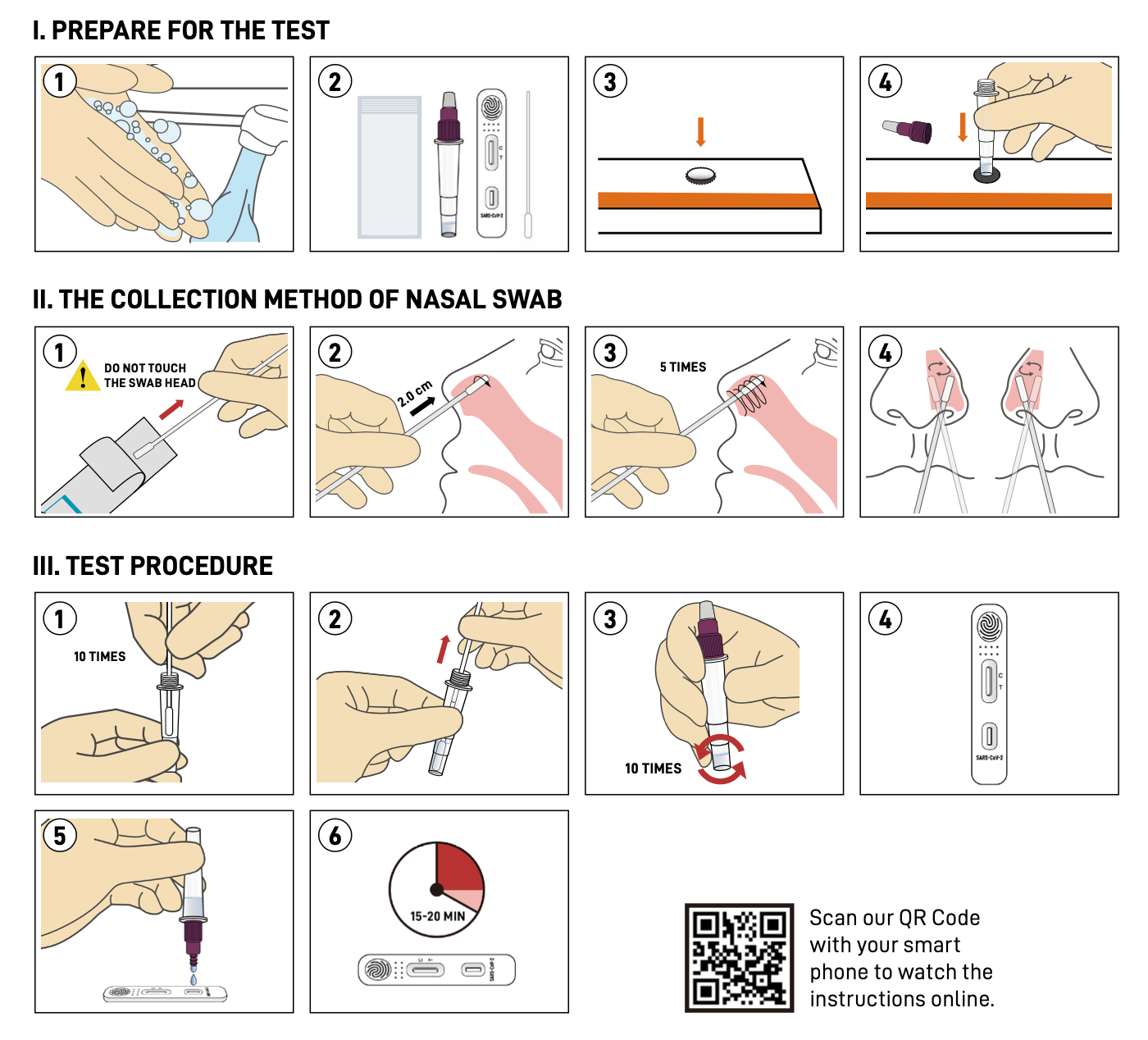
Result interpretation
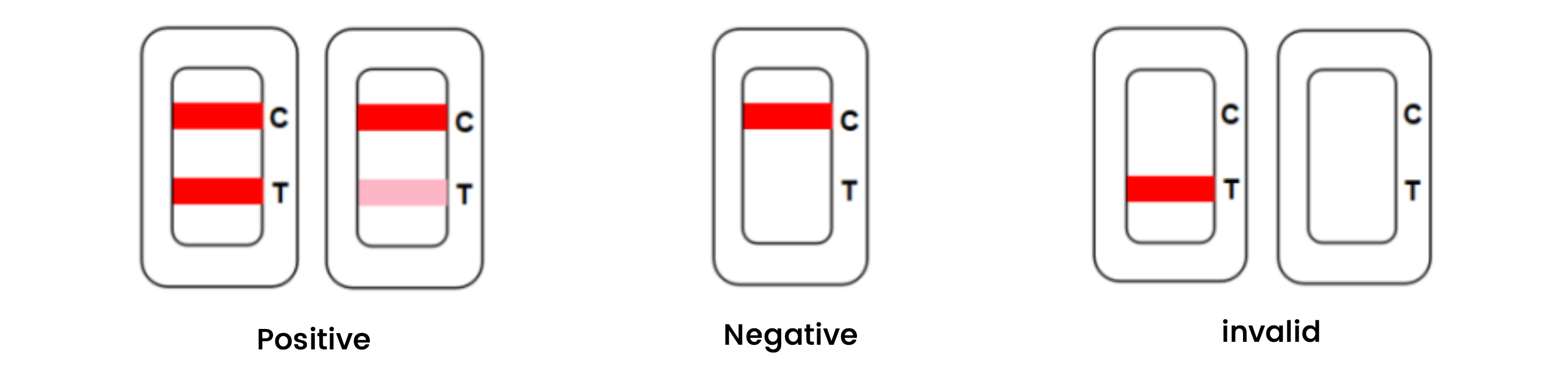
Parameters
Qualification CE Kit components
No. Product Name Spec. S3120E-1-H, S3120E-5-H SARS-CoV-2 Rapid Antigen Test (Immunochromatography Assay) 1 Test, 5 Tests The kit components:
- SARS-CoV-2-Antigen Test Cassette (with desiccant)
- SARS-CoV-2 Sample Extraction Buffer
- Swab
- Plastic Waste Bag
-

S3109E SARS-CoV-2 Rapid Antigen Test (Colloidal Gold Method)
Respiratory Tract InfectionsBrief
The SARS-CoV-2 Rapid Antigen Test (Colloidal Gold Method) is intended for the qualitative detection of the SARS-CoV-2 nucleocapsid protein in human nasopharyngeal or nasal swab sampled from individuals suspected of COVID-19.Parameters
Testing Time 10-15 minutes Sensitivity 98.4% Specificity 98.1% Qualification CE Kit components
No. Product Name Spec. S3109E-25 SARS-CoV-2 Rapid Antigen Test (Colloidal Gold Method) 25 tests -


iPonatic – Portable Molecule Workstation
mPOCT, PCR InstrumentsBrief
Innovations in molecular assays—especially on the point-of-care testing (POCT) front—have spread this testing from molecular diagnostics laboratories into clinical microbiology laboratories—and now into general laboratories and even clinics and exam rooms.[1] Access to sensitive and rapid infectious disease diagnostic assays is essential for accurate diagnosis, effective treatment, and timely infection control, making POCT vital to reducing TAT. With novel POCT on the horizon, future studies are warranted to determine cost savings, antimicrobial usage, TAT, patient impact, and how to best implement in non-microbiology clinical laboratories and clinics.[2] Sansure iPonatic (Portable Molecule Workstation) aims to innovate traditional diagnostic mode and assist precision diagnosis. It can provide quickly and convenient diagnosis experience for clinical emergency, health management, military safety, biological emergency and other applications.Core technologies
- Rapid nucleic acid lysis at room temperature within 1 minute
- Ultra fast amplification system in 8-45 minutes
- Integrated automatic data analysis software
- Results immediately printed by built-in printer
Performance
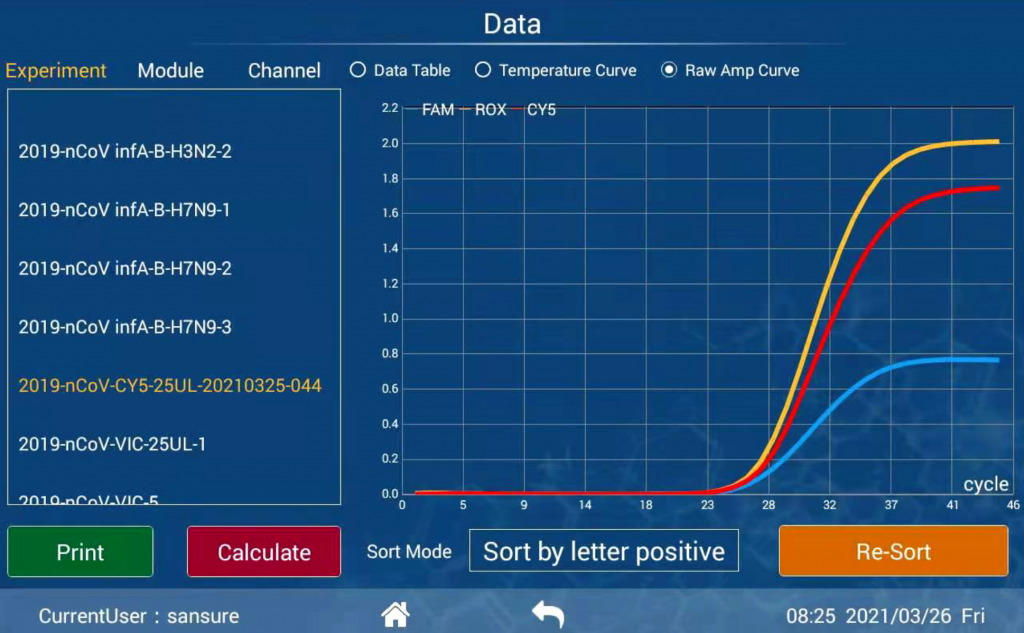
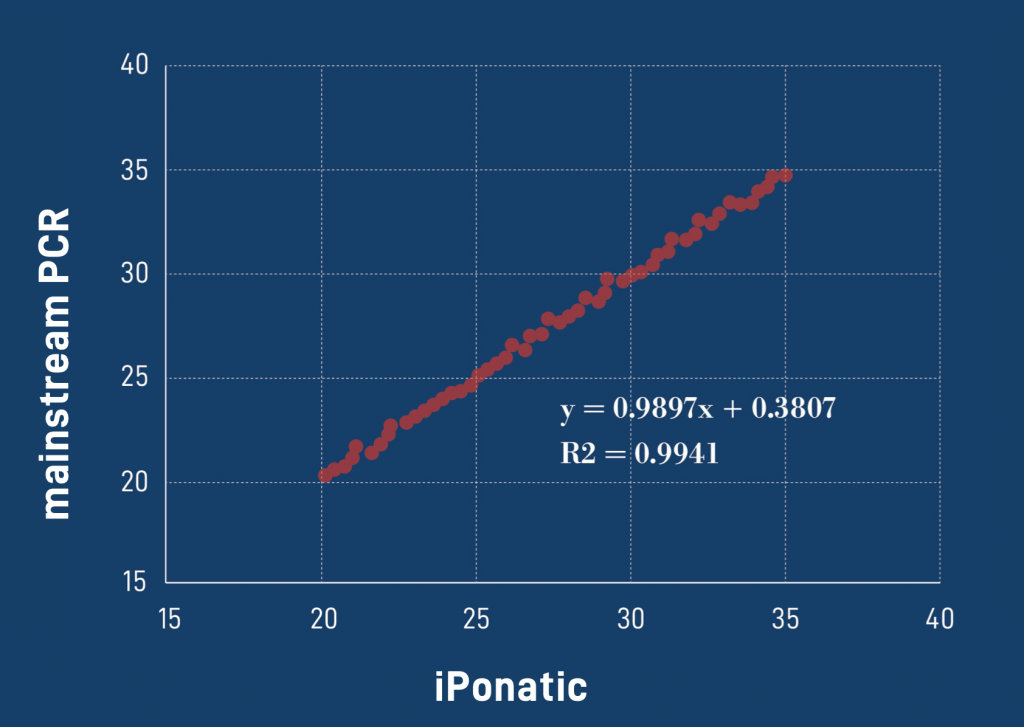
Diagnostic results consistent with international mainstream PCR instruments
Parameters
Model S-Q31A S-Q31B Detection platform Real-time PCR Detection module 1 amplification module 4 amplification modules Temperature control Liquid metal coated ceramic heating / Air cooling technology Heating rate ≥6.0℃ /s (from 50℃ to 95℃ ) Cooling rate ≥2.0℃ /s (from 95℃ to 50℃ ) Excitation light source LED Detector High sensitivity photodiode Applicable dyes FAM VIC ROX CY5 Sensitivity Can detect single copy gene Electrical specification AC 100-200V 50/60Hz Dimension 230×284×376 mm (L×W×H) 336.5 × 280 × 435 mm (L×W×H) Weight 9.7Kg 13Kg Qualification CE Test menu
Respiratory Infections : SARS-CoV-2, SARS-CoV-2/FluA/B, MP, ADV, BP.... HPV Infections : HrHPV, HPV 13+2, HPV 16/18, HPV 6/11.... Children's health : EBV, HCMV.... STIs : CT/UU/NG, CT, UU, NG, HSV-2.... Other test projects are under developmentReferences
[1]. Kozel TR, Burnham-Marusich AR. Point-of-care testing for infectious diseases: Past, present, and future. J Clin Microbiol 2017;55:2313-20.
[2]. Paige M.K. Larkin, Omai B. Garner. Molecular Point-of-Care Testing in Clinical Laboratories Laboratorians can lead a new era in rapid testing with expertise in quality control and result interpretation. Clinical Laboratory News. JUL.1.2020
-


MA-6000 Real-Time Quantitative Thermal Cycler
PCR InstrumentsBrief
The MA-6000 Real-Time Quantitative Thermal Cycler has spent many years in the research and development phase, but it was worth the wait as this advanced technology will help you treat more patients and cut down your waiting times with its ability to process up to 96 samples at one time. Our new Real-Time fluorescence quantitative PCR system MA-6000 is equipped with innovative hardware, structure and optimised software to deliver higher quality results.Features
Temperature Control Technology and Innovation MA-6000 employs a six independent temperature control module, associated with an infrared environmental scanning and monitoring system for temperature control and secured precision for the thermal block. A technologically designed thermal gradient customises twelve thermal conditions for multiple reaction actions in scientific research, maximising convenience for your research and scientific experiments. The MA-6000’s platinum sensor prevents temperature overshoot and undershoot, homogenised temperature variance which in tandem offer customers reliably excellent and repeatable data output. The World's Leading Optical Detection Technology’s Built-In Advantages A world leading technology of optical conduction and detection is applied to MA-6000. A highly functional heat-resistance optical fibre conducts full-spectrum halogen light source to samples without attenuation. The emission fluorescence signals are synchronously collected by monochrome CCD, which together with its cooling system, physically eliminate dark current on the detection array. MA-6000 ensures extraordinary detection sensitivity, extends the application of thermal cycler from nucleic acid to biotinylated protein and expands a brand-new path for multiplex diagnosis in clinical field.Specifications
PCR Module Sample capacity 96×200ul tubes / 12 x 8-well PCR strips / l x 96-well plate Temperature range 4-100 ℃ Maximum ramp rate 3.5 ℃/s for heating; 3.2℃/s for cooling Temperature accuracy ± 0. 1 ℃ Temperature uniformity ±0.25℃ Temperature monitoring method 6 independent zones for temperature surveillance Gradient capability Yes Excitation light source Full spectrum halogen lamp(5 years warranty) Excitation spectrum 380-780nm Excitation channels Built-in 6(including I extension channels) Fluorescence dyes/probes FAM / SYBR Green I / VIC / HEX / TET / Cy3 / Cy3.5 / JOE / Yellow 555 / ROX / Texas Red / Cy5 / Cy5.5 / LC Red / Tamara Detection channels 96 two-way heat-resistance optical fibers Data resolution 5.000-10.000 copies with 99.8% confidence; 1.5 times differentiation for single reaction Thermo Cycler Power supply 100-240V Frequency 50-60Hz Dimensions(WxHxD)(cm) 54.8×38.8×28.8 Weight 23kg Software Win7, Win8, Win10, etc. Qualification CE -


SLAN-96P Real-Time PCR System
PCR InstrumentsFeatures
Precise Temperature Control System: 1. The SLAN system adopts Peltier technology which can accurately and quickly control increases and decreases of temperature with a precision of ±0.1℃ and features long service life, no noise and no pollution, etc.; 2. Multiple-point temperature control technology ensures the uniformity between different wells. The temperature correction technology guarantees the accuracy of temperature and thus ensures the accuracy of assay result; 3. PCR tube temperature-control technology guarantees the accuracy of actual temperature of reagent in the tube and enables the SLAN to easily adjust for different reactions using different volumes. Sensitive Photoelectric Detection System 1. High sensitivity photoelectric detector with no background noise which is fast and stable; 2. Lifetime maintenance-free ultra-bright LED cold light source with a large signal value and high stability; 3. Unique optical fiber conduction technology that greatly improves fluorescence collection efficiency and avoids fluorescence interference between neighboring tubes; 4. The system automatically selects best gain value for each tube without the need for user configuration. The SLAN®has an extremely broad measuring range for fluorescence; 5. Extremely high detection sensitivity with an extremely low fluorescence background value. It's not necessary for users to make optical correction or background correction.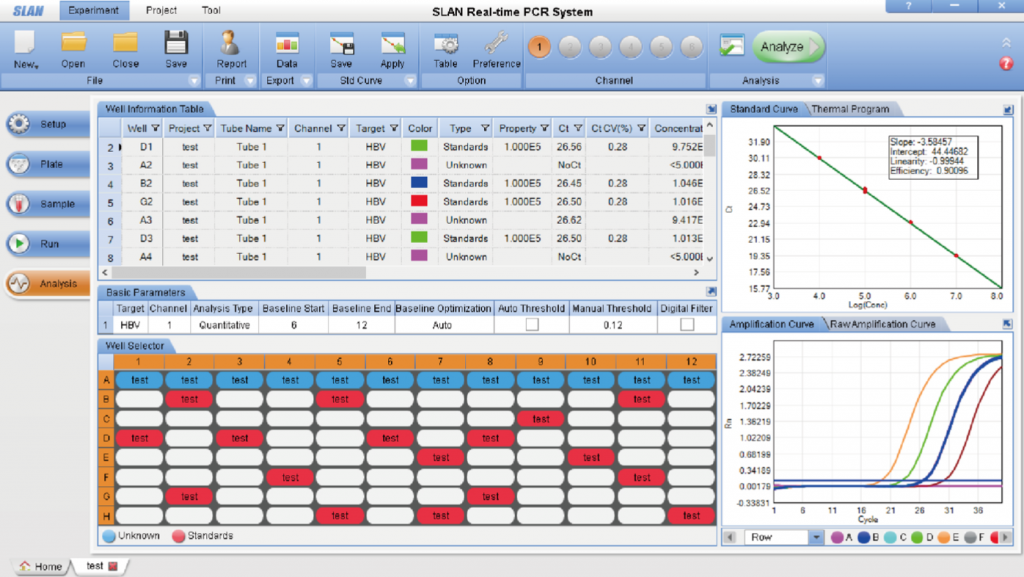
SLAN-96P Multi-tasking Software Interface (analysis screen)
Unique Electronic Automatic Hot Lid System 1. Flexible heating technology ensures uniform hot lid temperature; 2. The electronic automatic hot lid has an adjustment function for different 0.2ml reaction tubes to ensure even tube pressure; 3. Once the assay starts, the hot lid has automatic locking function to prevent assay failure following accidental opening. Other functions· Power-off protection · Automatic saving result · Export of result · Real-time data display · Quantitative and qualitative analysis · File encryption · Automatic baseline optimization · Digital filtering · Search function · Report sheet print and edit · Melt curve function Specifications
Product model SLAN-96P Sample capacity 96 wells (dual 48/48 reaction module) Sample size 15-50mL Consumable 0.2mL PCR tubes, 8-tube strips, 48-well plates Parallel running mode Dual reaction blocks/running 2 tests independently Temperature range 4-99°C Max ramp rate 4.0°C/sec Temperature accuracy ±0.1°C Temperature uniformity ±0.1°C Thermal cycling system Peltier-based system Temperature control mode Tube control/block control Light Source LED (maintenance-free) Detector High sensitivity photoelectric sensor Sensitivity 1 copy Linearity range 100-1010 Resolution Can discriminate between 1000 copies and 2000 copies Excitation CH1 470nm CH2 530nm CH3 580nm CH4 630nm CH5/CH6 custom-made Emission CH1 510nm CH2 565nm CH3 620nm CH4 665nm CH5/CH6 custom-made Dyes and probes CH1 FAMTM /SYBRGreen® CH2 VIC®/HEX/J0ETM /TET CH3 ROX/TexasRed® CH4 CY5TM Hot-lid 30°C~108°C (default105°C, adjustable) electronic automatic hot-lid Power supply 230VAC, 50Hz Power consumption 850VA Dimensions 380mm x 520mm x 250mm (WxDxH) Weight 18Kg Communication RS232, USB Qualification CE -


Natch 48 Nucleic Acid Extraction System
Extraction InstrumentsExtremely simple workflow

Specifications
Instrument model Natch 24/48/96 Sample type Serum, plasma, whole blood, secretions, exfoliated cells, tisues, throat swabs, anal swabs, urine, feces, etc. Extraction technology Advanced magnetic beads technology Sample type Volume 1-96 tests/batch Extraction Time 10-60 minutes each batch Working volume 30-2000uL Recovery rate of magnetic beads >95% Blending mode Vortex omnidirectional liquid mixing Temperature control range Adjustable from roomtemperature to 125℃ Operation 7-inch touch screen Storage Up to 120 groups of programs can be used. Size and weight 655x655x520 mm (L x W x H), weight: 65kg Qualification CE Extraction kit components
No. Spec. Product Name S10016E 48T/kit Nucleic Acid Extraction-Purification Kit (Magnetic beads method) Available projects
Sexually Transmitted Diseases : CT, NG, UU, HSV-2, HSV I/2 Women's Health : hrHPV and hrHPV genotyping Respiratory Tract Disease : 6RP, TB, MP, RSV, ADV, BP Gastrointestinal Diseases : HFMD Prenatal and Postnatal care : CMV, EBV Public Health : SARS-CoV-2, Influenza A/B, HINI, H7N9 Customerized Projects -


Natch CS2 Fully Automated Nucleic Acid Extraction System
Extraction InstrumentsExtremely simple workflow

Features
1. Efficient anti-contamination
2. Super magnetic, effective adsorption
- Well-organized countertop layout
- Sample area is separated from clean area
- Effective UV decontamination
- Anti-contanmination function of moving path
3. Excellent sample addition function
- Super 3-D magnetic adhesion technology
- Permanent magnetic mode
- 117 magnetic racks, 4 magnetic bars in each well
4. Easy-to-use software
- Automatic liquid level detection
- Clot detection
- Tip detection
- Air-prof detection
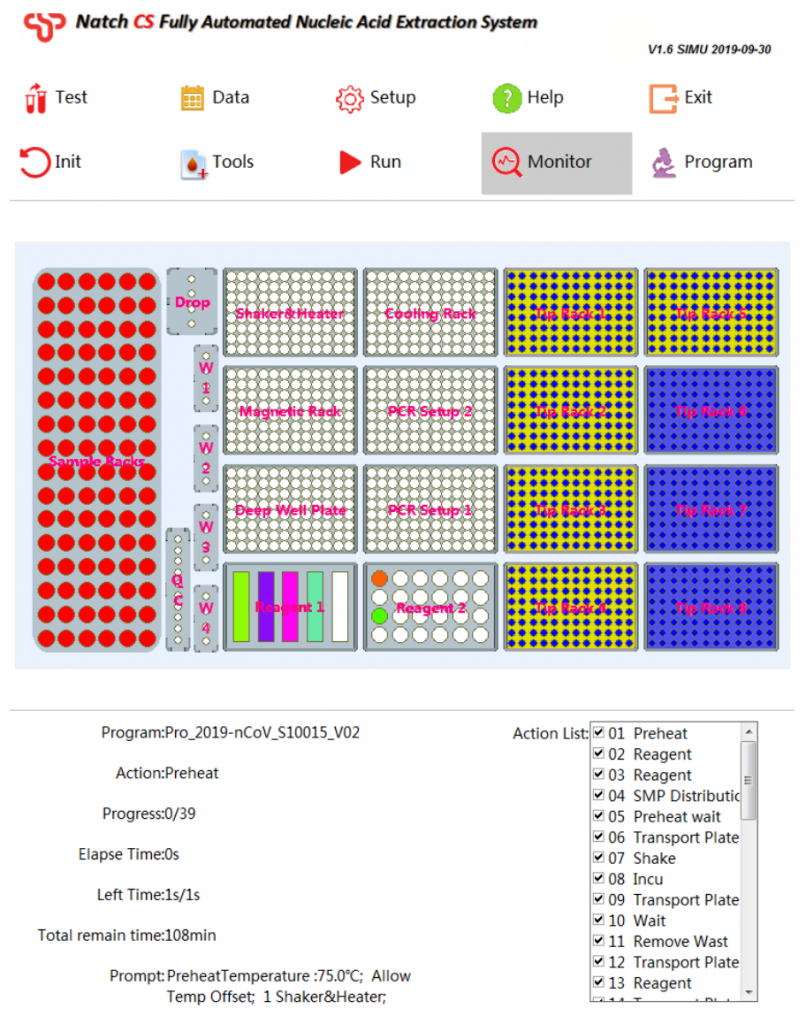
- Preset programs, one-key selection
- Prompt the placement of reagents
- Display the status of reagents and consumables
- Real-time display of remaining time
- Show real-time experimental procedures
- Permits LIS compatibility
Specifications
Instrument model S-S13A Sample type Serum, plasma, whole blood, secretions, exfoliated cells, tissues, nasopharyngeal swab, oropharyngeal swab, urine, feces, etc. Sample tube specifications Compatible with various original sample tubes Extraction Principle one-tube fast release technology (OT) Advanced magnetic beads technology (MB) Sample type Volume OT: 1-96 tests/batch; MB: 1-96 tests/batch Extraction Time OT of 96 tests ≤ 30 minutes; MB of 96 tests ≤ 90 minutes Pipetting range 10~1000 μL Level detection Pressure detection / capacitance detection, automatic detection of liquid level Clot detection Pressure detection, automatic detection of liquid aspiration and liquid clogging Sample loading function Automatic liquid level detection Clot detection Tips detection Air-prof detection Magnetic field control Permanent magnet mode Patented design Super 3-D magnetic adhesion technology Blending mode Vortex omnidirectional liquid mixing Temperature control range Adjustable from room temperature to 100℃ UV disinfection Timed opening and closing Barcode Scanning Sample scanning system Supported multiple barcodes Language Simplified Chinese / English Display Method 10.4 inches touch screen Communication Interface USB, RS232, network ports, HDMI Dimensions and Weight 1320×850×1600mm (LxWxH), weight: 350Kg Power supply Input: AC 100-240V 50/60Hz 350 VA Qualification CE Extraction kit components
No. Spec. Product Name S1013E 48T/kit Sample Release Reagent S1014E 24T/Kit, 48T/kit Sample Release Reagent S1002E 24T/kit Nucleic Acid (DNA/RNA) Extraction or Purification Kit (Magnetic beads method) S1006E 48T/kit Multi-type Sample DNA/RNA Extraction-Purification Kit (Magnetic beads method) S10016E 48T/kit Nucleic Acid Extraction-Purification Kit (Magnetic beads method) Available projects
Hepatitis and AIDS : HBV, HCV, HIV Sexually Transmitted Diseases : CT, NG, UU, HSV-2, HSV I/2 Women's Health : hrHPV and hrHPV genotyping Respiratory Tract Disease : 6RP, TB, MP, RSV, ADV, BP Gastrointestinal Diseases : HFMD Prenatal and Postnatal care : CMV, EBV Public Health : SARS-CoV-2, Influenza A/B, HINI, H7N9 Customerized Projects -


S1006E Multi-type Sample DNA/RNA Extraction-Purification Kit
DNA/RNA Extraction ReagentsBrief
Multi-type Sample DNA/RNA Extraction-Purification Kit (Suprall extraction reagent) is an innovative product based on Sansure advanced magnetic beads technology platform , which can accomplish the unified extraction of nucleic acids in various gene detection applications and samples to provide comprehensive solutions for nucleic acid extraction , can be applied in infectious disease diagnosis, genetic disease screening, birth defect prevention, tumor screening, individualized diagnosis and treatment, detection of disease susceptibility genes, sequencing services and other fields.Parameters
Qualification CE Kit components
No. Product Name Spec. S1006E Multi-type Sample DNA/RNA Extraction-Purification Kit 24T/kit -

Nucleic Acid (DNA/RNA) Extraction or Purification Kit
DNA/RNA Extraction ReagentsBrief
Nucleic Acid (DNA/RNA) Extraction or Purification Kit developed based on Sansure advanced magnetic beads technology platform,using exclusive modified super-paramagnetic nano-beads to absorb sample DNA/RNA , only needs a simple step of washing to obtain high purity nucleic acid, combined with the "DNA/RNA elution-free " technology and integrated high-efficient amplification system, to achieve DNA/RNA amplification detection with magnetic beads.Parameters
Qualification CE Kit components
No. Product Name Spec. S1002E Nucleic Acid (DNA/RNA) Extraction or Purification Kit 24T/kit S10011E Nucleic acid (DNA/RNA) extraction or purification kit 24T/Kit, 48T/kit S10016E Nucleic Acid Extraction-Purification Kit 48T/kit, 96T/kit -


Sample release reagent
DNA/RNA Extraction ReagentsBrief
Sample release reagent developed based on the Sansure one-tube fast release technology platform. Adopting Sansure patent nucleic acid release technology, can quickly lyse pathogens at room temperature, no need heating, centrifuging or replacing tubes, the sample DNA/RNA can be extracted quickly through simple operations. Sample Release Reagent is used for the pretreatment of nucleic acids, to release the nucleic acids from specimens, then the released nucleic acids can be used for clinical in vitro diagnosis or used for the detection through equipment.Parameters
Qualification CE Kit components
No. Product Name Spec. S1011E Sample Release Reagent 48T/kit S1013E Sample Release Reagent 48T/kit S1014E Sample Release Reagent 24T/Kit, 48T/kit -


S3053E EV/EV71/CA16 – Enterovirus/Coxsackievirus A16/Enterovirus 71 RNA Diagnostic Kit
Gastrointestinal Infections (GIIs)Brief
Hand-foot-and-mouth disease is a common childhood disease caused by enteroviruses. Most of the patients will have a fever and get vesicle rash in the mouth or on the hand and foot. A few patients will get cephalomeningitis, cerebritis, neurogenic pulmonary edema and myocarditis, etc. Some patients may get sequelae of this disease or even death. The viruses that can cause hand-foot-and-mouth disease include Coxsackievirus, new-type enterovirus and ECHO virus, of which the most common types are Coxsackievirus A16 and Enterovirus 71. The laboratory diagnosis methods mainly include virus isolation, nucleic acid detection, etc. The Enterovirus/Coxsackievirus A16/Enterovirus 71 RNA Diagnostic Kit (PCR-Fluorescence Probing) is intended to detect Enterovirus, Coxsackievirus A16 and Enterovirus 71 in throat swab by applying real-time quantitative PCR technique. The detection results can be used to distinguish Coxsackievirus A16 and Enterovirus 71.Parameters
Product features Parameter Specimen Type throat swab Technical Platform Advanced magnetic beads technology Target pathogen EV, EV71, CA16 Internal Control Lentivirus PCR Instrument ABI7500,SLAN-96P Amplification Time 100 min Sensitivity 1000 copies/mL Spec. 24T Qualification CE -


S3014E HCMV – Human Cytomegalovirus DNA Quantitative Fluorescence Diagnostic Kit
Other InfectionsBrief
Human cytomegalovirus (HCMV), also called cell inclusion body virus, is a double helix DNA virus and belongs to β genus of herpes virus family, which causes infected cells to enlarge and has a huge intranuclear inclusion. The ways of infection of HCMV is mainly through contact, blood transfusion, intrauterine and birth canal, and the infections are commonly found in fetus, newborns, pregnant women, etc. If the pregnant women are infected, it may cause their newborns congenital monstrosity. When the organism immune deficiency or immune system is under inhibitory state, people can be easily infected by HCMV, such as the patients receiving immunosuppressive therapy after transplantation of organ, the patients receiving malignant tumor chemotherapy and AIDS patients, etc. If these patients are infected by HCMV, it usually causes high mortality and serious diseases. Clinical tests suggest that HCMV infection hasn’t specific manifestations and it causes harm to multiple organs, especially the liver and lung. A statistical analysis of positive and negative rate of HCMV in various kinds of clinical indications shows HCMV infection is probably related with various diseases, such as Cytomegalovirus hepatitis, Infant hepatitis syndrome, liver dysfunction, pneumonia, Bronchitis, Upper respiratory tract infections, enteritis, enterocolitis, diarrhea, hematemesis, heart failure, etc. The Human Cytomegalovirus DNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of HCMV DNA in human urine, serum and peripheral blood samples. It is intended for use as an aid in the diagnosis of an HCMV infection and for observing drug efficacy.Parameters
Product features Parameter Specimen Type urine, serum, and peripheral blood. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen HCMV Internal Control cloning plasmid PCR Instrument ABI7500, Roche LC 480, Stratagene Mx3000P, SLAN-96P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T Qualification CE -


S3015E EBV – Epstein-Barr virus DNA Quantitative Fluorescence Diagnostic Kit
Other InfectionsBrief
Epstein-barr virus (EBV) is known to be the first virus that is definitely related with human tumors. EBV infection mainly causes infectious mononucleosis in children and tumor-related diseases such as Burkitt’s lymphoma, lymphoid tissue hyperplasia in immunocompromised individuals, primary lymphoma, empyema associated lymphoma, T-cell lymphoma, NK cell lymphoma/leukemia, Hodgkin’s disease, nasopharyngeal carcinoma, stomach cancer, lymphoid epithelial tissue cancer, smooth muscle tumors, etc. The Epstein-Barr virus DNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the quantification of Epstein-Barr Virus (EBV) DNA in peripheral blood. Test result can be used as an aid in the diagnosis of an EBV infection and in observation of drug efficacy.Parameters
Product features Parameter Specimen Type peripheral blood. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen EBV Internal Control cloning plasmid PCR Instrument ABI7500, Roche LC 480 and Stratagene Mx3000P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T Qualification CE -

C003E BCI – Nucleic Acid Test Kit for HBV, HCV, HIV (Type1+2)
Blood-borne Infections (BBIs)Brief
Nucleic Acid Test Kit for HBV, HCV, HIV (Type1+2), is based on real-time fluorescence PCR technology and used for nucleic acid qualitative detection of HBV, HCV, HIV1+2 in plasma. This kit is intended for use as a blood donor screening test to detect HBV DNA, HCV RNA, HIV-1 RNA and HIV-2 RNA in pooled or individual sample from healthy blood donors, blood donors of various components (red blood cells, platelets and plasma) and other types of blood donors. All plasma to be tested can be screened as individual samples or tested in pools after mixing with each equal aliquots according to routine serological screening results HBV, HCV and HIV samples. For the pooled sample showed positive test results, carry out individual testing, the individual test result should be used as the final test result of this sample. The test results of this diagnostic kit can distinguish reactivity of between HBV, HCV and HIV.Advantages
High sensitivity- HBV 3IU/mL
- HCV 10IU/mL
- HIV 45IU/mL
- 576 tests/ 5h(pooling)
- 45 tests / 4.5h(single
- HBV A-H
- HCV 1-6
- HIV-1(M/N/O)and HIV-2
- Four tests for a tube of samples
- Direct discriminating positive
- Extraction and amplification process
- No need to be on duty
- One extractor + one amplifier
- Less consumable material consumption
Features
Sample preparation and extraction in one module Fully automatic sample preparation and nucleic acid extraction in one module to build up a integrated reaction system Flexible testing mode 6 pooling sample testing or individual donor testing (IDT) supported Advanced magnetic beads technology Nanometer-level beads enable beads-in-PCR amplification ensuring maximum nucleic acid template Patented magnetic beads lateral suction technology Thorough waste liquid removal allows minimal magnetic beads loss Sophisticated sample information processing Automatic recognition of the sample barcode & sample tracking and archiving table generation available High throughput 558/45 samples result output in one time Minimal system maintenance time Less than 20 minutes startup with fewer maintenance tasks Excellent accuracy A total number of 105,124 blood bags which confirmed as negative by serological testing were tested against some referential screening kits. 15 false-negative results in reference tests were found.Parameters
Qualification CE -

S3034E HCV Genotype – Hepatitis C Virus Genotype Diagnostic Kit
Blood-borne Infections (BBIs)Brief
By applying real-time PCR technology, the Hepatitis C Virus Genotype Diagnostic Kit (PCR-Fluorescence Probing) is designed for qualitative identification of HCV genotypes (including genotypes 1b, 1, 2, 3, 4, 5 and 6) from HCV RNA positive samples. The test results can be used as an aid in the identification of HCV genotypes and determination of an appropriate therapeutic treatment indicated for the above listed gentoypes. The results can be used only for clinical reference, and cannot be used as the only evidence for adjusting therapeutic drugs. Clinical symptoms and other laboratory test results should also be considered to comprehensively determine the patients treatment.Parameters
Product features Parameter Specimen Type serum Extraction Platform Magnetic beads technologies Genotype 1b, 1(1b, 1a), 2 (2a), 3 (3a, 3b), 4 (4a), 5 (5a), 6 (6a) Internal Control Plasmid PCR Instrument Mx3000P,Slan 96P Sensitivity 1000 IU/mL Spec. 12T Qualification CE -


S3119E HCV Ultra – Hepatitis C Virus RNA Quantitative Fluorescence Diagnostic Kit
Blood-borne Infections (BBIs)Brief
Hepatitis C Virus RNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the quantification of HCV RNA in human serum or plasma. It is intended for use as an aid in the diagnosis of an HCV infection and observing drug efficacy. Hepatitis C is mainly caused by HCV infection and transmitted through blood. Chronic infection of HCV can lead to chronic inflammation of liver and fibrosis, and some patients may develop into liver cirrhosis, even Hepatocellular Carcinoma (HCC). It has huge harms on patients’ health and life quality, and has become a severe social and public health issue.Parameters
Product features Parameter Specimen Type Serum or plasma Extraction Platform Advanced magnetic beads technology Genotype HCV 1-6 genotype Internal Control Lentivirus particles PCR Instrument SLAN-96P, ABI7500, Roche cobas 480 Sensitivity 12 IU/mL Linear range 25—1.0E+08 IU/mL Spec. 24T -

S3013E HBV fast – Hepatitis B Virus DNA Quantitative Fluorescence Diagnostic Kit
Blood-borne Infections (BBIs)Brief
Hepatitis B Viral (HBV) DNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the quantification of HBV DNA in human serum or plasma. It can be used to evaluate antiviral treatment and monitor the therapeutic effect by monitoring HBV DNA baseline levels and changes in patient blood. Test results should not be taken as the only indicator for evaluation of diseases, but to be combined with patients clinical symptoms and other laboratory tests to analyze the diseases.Parameters
Product features Parameter Specimen Type Serum or plasma Extraction Platform One-tube fast test release Genotype HBV genotype A-H Internal Control Plasmid Compatible Instrument ABI7300 , Stratagene Mx3000P , Roche Light Cycler 480 , ABI7500, SLAN-96P and QuantStudio 5 Sensitivity 30 IU/mL Linear range 100—5.0E+09 IU/mL Spec. 24T, 48T -

S3118E HBV Ultra – Hepatitis B Virus DNA Quantitative Fluorescence Diagnostic Kit
Blood-borne Infections (BBIs)Brief
Hepatitis B Virus DNA Quantitative Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) (HBV Ultra) is an in vitro nucleic acid amplification test for the quantification of Human HBV DNA in human serum or plasma. It is intended for use as an aid in diagnosing an HBV infection and observing drug efficacy.Performance
Advanced magnetic beads technology HBV DNA detection and viral load measurement are essential for treatment decisions and patient monitoring. Sansure's HBV Ultra uses the advanced magnetic beads technology to extract HBV DNA from clinical Plasma. Our technology achieved high sensitivity and wide linear range detection to meet of clinical diagnosis and follow-up needs. Highly conservative primer probe design Sansure primers and probes target select the highly conservative S gene of HBV, which can cover A-H genotypes and avoid missed detection. High-efficient quality control system HBV Ultra uses UNG enzyme + dUTP system to remove carry-over contamination to avoid a false positive result. HBV Ultra uses Internal Control HBV Ultra uses Internal Control is the full name to whole-process the HBV extraction and amplification process to avoid false negative results.Parameters
Product features Parameter Specimen Type Serum or plasma Extraction Platform Advanced magnetic beads technology Genotype HBV genotype A-H Internal Control Pseudoviruses PCR Instrument SLAN-96P, ABI7500, Roche cobas 480 Sensitivity 5 IU/mL Linear range 20—2.0E+09 IU/mL Spec. 48T -

S3017E HPV 6,11 – Human papillomavirus (Type 6 and 11) DNA Fluorescence Diagnostic Kit
HPV InfectionsBrief
HPV (Type 6 and 11) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of low-risk HPV (type 6/11) DNA in reproductive tract secretions. It is intended for use as an aid in the diagnosis of an HPV (type 6/11) infection and patients with clinically suspected genital warts.Parameters
Product features Parameter Specimen Type reproductive tract secretions Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 6, 11 Internal Control β--globin gene PCR Instrument ABI7500,QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II,iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -

S3019E HPV 16, 18 – Human papillomavirus (Type 16 and 18) DNA Fluorescence Diagnostic Kit
HPV InfectionsBrief
HPV (Type 16 and 18) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (Type 16 and 18) DNA in reproductive tract secretions. It is intended for use as an aid in the diagnosis of an HPV (Type 16 and 18) infection, and the early screening of cervical cancer.Parameters
Product features Parameter Specimen Type reproductive tract secretions Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 16, 18 Internal Control β--globin gene PCR Instrument ABI7500,QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II,iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -

S3360E HPV 15HR – 15 High-risk Human Papillomavirus Nucleic Acid Diagnostic Kit
HPV InfectionsBrief
15 High-risk HPV DNA Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68) present in exfoliated cells from females’ cervix. The test results can be used as an aid in the diagnosis of a high-risk HPV infection.Parameters
Product features Parameter Specimen Type exfoliated cells from females’ cervix Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68 Internal Control β--globin gene PCR Instrument ABI7500, QuantStudio 5, MA-6000, SLAN-96P, LightCycler 480, Stratagene Mx3000P, iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 1000 copies/mL Spec. 48T, 12-P Qualification CE -


S3027E HPV G15 – High-risk Human Papillomavirus DNA (Genotype) Diagnostic Kit
HPV InfectionsBrief
This High-risk HPV DNA (Genotype) Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68) present in exfoliated cells from females’ cervix. The test results can be used for identification of HPV genotypes.Parameters
Product features Parameter Specimen Type Exfoliated cells from females’ cervix Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68 Internal Control β--globin gene PCR Instrument SLAN-96P, ABI 7500, Roche LC 480 and QuantStudioTM 5 , Stratagene Mx3000P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 24T/48T Qualification CE -


S3057E HPV 13+2 – Human Papillomavirus DNA Diagnostic Kit
HPV InfectionsBrief
Base on the study results by WHO International Agency for Research on Cancer (IARC) and other international organizations, the 13 kinds of genotypes including HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 are classified as high-risk HPV, and the 5 kinds of genotypes including HPV26, 53, 66, 73, 82 are classified as medium-risk HPV. Human Papillomavirus DNA Diagnostic Kit (PCR-Fluorescence Probing) chooses the above 13 kinds of high-risk genotypes and two popular kinds of medium-risk genotypes that are HPV 53 and 66 to be the target genotypes, in order to guarantee that the kit is capable of cervical carcinoma screening and risk evaluation. Moreover, it is clearly indicated that the females at the age of 30 or above who have no abnormal cervical cytology but the HPV detection is positive, especially for HPV16 and HPV18 infected females, should get vaginoscopy immediately. Therefore, the diagnostic kit can be used for detection of the 15 kinds of target genotypes and also subtype identification of HPV16 and HPV18.Parameters
Product features Parameter Specimen Type Exfoliated cells from females’ cervix Technical Platform One-tube fast release technology Advanced magnetic beads technology Detection types Type 16+18+31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68 Internal Control β--globin gene PCR Instrument ABI7500, MA-6000, SLAN-96P, QuantGene 9600 , iPonatic S-Q31A&B, S-Q36A Amplification Time 70 min Sensitivity 200 copies/mL Spec. 48T, 24-P Qualification CE
